High-Throughput Multiplexed Quantification of Molecules by Aptamer Sequencing (Apt-seq) in Single Cells
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The advent of high-throughput sequencing technologies is transforming life sciences into a quantitative paradigm. However, existing sequencing technologies for nondirectly sequencable molecules, such as antibody-sequencing and glycan-sequencing, face certain challenges, including the complexity of antibody-oligonucleotide conjugation procedures, potential steric hindrance, and the labor-intensive chemoenzymatic labeling. Aptamers, as directly sequencable nucleic acids, offer exceptional specificity, broad target range, and small size, making them ideal tools for multimodal molecular profiling. In this work, we present Apt-seq, an aptamer-based, high-throughput platform for multimodal omics quantification at single-cell resolution. This integrative strategy─termed Aptomics─enables parallel profiling of cell surface proteins, glycans, and mRNA. The feasibility of the platform was validated using commercial cell lines, demonstrating strong concordance between sequencing data and flow cytometry results at both bulk and single-cell levels. When applied to complex clinical samples, Apt-seq exhibited exceptional sensitivity and enabled the precise profiling of tumor heterogeneity. Notably, the platform identified a subpopulation of tumor cells with elevated PTK7 surface expression, a marker of stemness. These findings underscore the role of PTK7 in tumor stemness and its potential as a stem-like biomarker. Moreover, using an aptamer targeting sialic acid, we tracked the dynamic changes in sialylation during T cell differentiation, observing an increase in sialic acid levels as resting T cells transitioned into functional T cells, followed by a subsequent decline upon maturity. Collectively, we have developed a new form of multiomics, termed Aptomics, which employs aptamers to enable the sequencing of molecules that are not directly sequenceable, such as proteins and glycans, etc. Apt-seq enables the simultaneous quantitation of both directly and nondirectly sequencable molecules, offering a versatile and scalable platform for the comprehensive quantification of molecules of life in complex biological systems and advancing quantitative science.
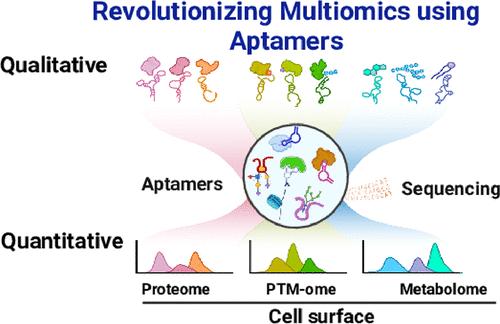
单细胞适体测序(Apt-seq)的高通量多路分子定量研究
高通量测序技术的出现正在将生命科学转变为定量范式。然而,现有的非直接可测序分子的测序技术,如抗体测序和聚糖测序,面临着一定的挑战,包括抗体-寡核苷酸偶联程序的复杂性、潜在的空间位阻和劳动密集型的化学酶标记。适体作为一种可直接测序的核酸,具有特殊的特异性、广泛的靶标范围和小尺寸,使其成为多模态分子分析的理想工具。在这项工作中,我们提出了Apt-seq,一个基于适配体的高通量平台,用于单细胞分辨率的多模态组学定量。这种整合策略──称为Aptomics──能够对细胞表面蛋白、聚糖和mRNA进行平行分析。利用商业细胞系验证了该平台的可行性,证明测序数据和流式细胞术结果在批量和单细胞水平上都具有很强的一致性。当应用于复杂的临床样本时,Apt-seq表现出异常的敏感性,并能够精确地分析肿瘤异质性。值得注意的是,该平台发现了一个肿瘤细胞亚群,其PTK7表面表达升高,这是干细胞的标志。这些发现强调了PTK7在肿瘤干性中的作用及其作为干样生物标志物的潜力。此外,利用针对唾液酸的适体,我们追踪了T细胞分化过程中唾液化的动态变化,观察到唾液酸水平在静止T细胞转变为功能性T细胞时增加,随后在成熟后下降。总的来说,我们已经开发了一种新的多组学形式,称为Aptomics,它使用适体来对不能直接测序的分子进行测序,如蛋白质和聚糖等。Apt-seq能够同时定量直接和非直接测序的分子,为复杂生物系统中生命分子的全面定量和推进定量科学提供了一个多功能和可扩展的平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


