Electronic states and fermiology of H3Ge and Li3Ge
IF 3.9
Q3 PHYSICS, CONDENSED MATTER
引用次数: 0
Abstract
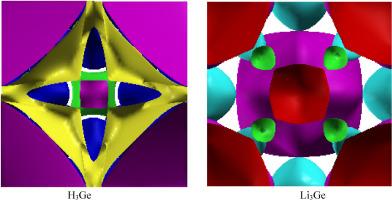
In this paper ab-initio characterization of electronic states, Fermiology and bonding of H3Ge and Li3Ge are presented. The Full Potential Linearized Augmented Plane Wave method is applied to study the structural properties, the vacancy migration enthalpy HM and lattice dynamics. The electronic properties such as band structure, density of states, directional Compton profiles and Fermi surface are presented. The low value of HM; 0.43 and 0.41 eV for H3Ge and Li3Ge, respectively, suggests faster diffusion, high atomic mobility and fast ion transport. The phonon dispersion of Li3Ge does not deliver imaginary frequencies. The electronic states reveal the metallic nature of both compounds. The comparison of band structure of H3Ge with that of synthetic H-sublattice reveal that Ge gives rise to the formation of bands around Fermi energy and responsible for the metallic behavior. In H3Ge the Fermi surfaces constituted by two bands are electron-like while in Li3Ge three bands constitute the Fermi surfaces which are hole-like. The quantum oscillations determined using de Haas Van Alphen effect shows fine features of the Fermi surface. Directional Compton profiles show more anisotropy and occupied states in Li3Ge than H3Ge. The J110-J100 anisotropy is higher than J111-J100 in both compounds. These observations are well captured by the branches of FS of the compounds. The auto correlation function gives 3.61 and 7.24 a.u bond lengths of H-H, Ge-Ge bonds in H3Ge. Similarly 4.48 and 8.96 a.u. bond lengths are obtained for Li-Li and Ge-Ge bonds in Li3Ge. The Ge-Ge, H-H/Li-Li interactions dominate in formation of bands and bonds in the two compounds.
H3Ge和Li3Ge的电子态和术语
本文介绍了H3Ge和Li3Ge的电子态、费米学和成键的从头算表征。应用全势线性化增广平面波方法研究了其结构性质、空位迁移焓HM和晶格动力学。给出了带结构、态密度、定向康普顿分布和费米表面等电子特性。HM值低;H3Ge和Li3Ge分别为0.43和0.41 eV,表明扩散更快,原子迁移率高,离子输运快。Li3Ge的声子色散不提供虚频率。电子态揭示了这两种化合物的金属性质。H3Ge的能带结构与合成h亚晶格的能带结构的比较表明,Ge在费米能周围形成能带,并对金属行为负责。在H3Ge中,由两个带组成的费米面是类电子的,而在Li3Ge中,三个带组成的费米面是类空穴的。利用德哈斯范阿尔芬效应测定的量子振荡显示出费米表面的精细特征。定向康普顿剖面显示Li3Ge比H3Ge具有更多的各向异性和占位态。J110-J100各向异性均高于J111-J100。这些观察结果被化合物的FS分支很好地捕获。自相关函数给出H3Ge中H-H、Ge-Ge键的键长分别为3.61和7.24 a.u。Li3Ge中Li-Li和Ge-Ge键的键长分别为4.48和8.96 a.u.。在这两种化合物中,Ge-Ge、H-H/Li-Li相互作用主导了能带和键的形成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Computational Condensed Matter
PHYSICS, CONDENSED MATTER-
CiteScore
3.70
自引率
9.50%
发文量
134
审稿时长
39 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


