Concentration-dependent structural transition of huntingtin protein in Huntington's disease
IF 2.2
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Huntington's disease (HD) is a genetic neurodegenerative disorder caused by the abnormal expansion of the polyglutamine (polyQ) tract (> 35Q) in the first exon of the huntingtin (Htt), HttEx1. This N-terminal fragment tends to form fibrillar inclusions, which constitute a key pathological hallmark of HD. Although polyQ expansion is commonly understood to be a primary cause of HttEx1 pathology, the molecular mechanism of aggregations of non-pathogenic polyQ tract with the N-terminally flanking region of N17 in HttEx1 (HttEx1-17Q) remains largely unknown. In this study, we exclusively investigated the effect of the protein concentration on the structural transition of HttEx1-17Q and its relation to the amyloid fibril formation by employing biophysical techniques including nuclear magnetic resonance (NMR) and circular dichroism (CD) spectroscopy, transmission electron microscopy (TEM), atomic force microscopy (AFM), and thioflavin T (ThT) fluorescence. Complementary analyses showed that monomeric HttEx1-17Q undergoes a multiple structural transition from largely unfolded structures to β structures via helical structures in a concentration-dependent manner in the early stages of aggregation. This structural rearrangement accelerates kinetically the formation of short amyloid fibrils of HttEx1-17Q by facilitating nucleation. Our findings provide new insights into the amyloid formation of HttEx1 by highlighting the critical role of a structural conversion into an amyloidogenic structure, of which mechanism is helpful to understand amyloidogenesis of other amyloid-forming molecules.
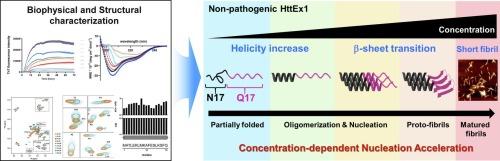
亨廷顿病中亨廷顿蛋白的浓度依赖性结构转变
亨廷顿氏病(HD)是一种遗传性神经退行性疾病,由聚谷氨酰胺(polyQ)通道异常扩张引起。35Q)在亨廷顿蛋白(Htt)的第一个外显子,HttEx1。这个n端片段倾向于形成纤维状包裹体,这是HD的一个关键病理标志。虽然多q扩增通常被认为是HttEx1病理的主要原因,但非致病性多q通道与HttEx1中N17的n端侧翼区域聚集的分子机制(HttEx1- 17q)仍然很大程度上未知。在这项研究中,我们专门研究了蛋白质浓度对HttEx1-17Q结构转变的影响及其与淀粉样蛋白纤维形成的关系,包括核磁共振(NMR)和圆二色(CD)光谱,透射电子显微镜(TEM),原子力显微镜(AFM)和硫黄素T (ThT)荧光。互补分析表明,单体HttEx1-17Q在聚集的早期阶段以浓度依赖的方式经历了从大部分未展开结构到螺旋结构的多重结构转变。这种结构重排通过促进成核,在动力学上加速了HttEx1-17Q短淀粉样原纤维的形成。我们的研究结果通过强调结构转化为淀粉样蛋白结构的关键作用,为HttEx1的淀粉样蛋白形成提供了新的见解,其机制有助于理解其他淀粉样蛋白形成分子的淀粉样蛋白形成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biophysical chemistry
生物-生化与分子生物学
CiteScore
6.10
自引率
10.50%
发文量
121
审稿时长
20 days
期刊介绍:
Biophysical Chemistry publishes original work and reviews in the areas of chemistry and physics directly impacting biological phenomena. Quantitative analysis of the properties of biological macromolecules, biologically active molecules, macromolecular assemblies and cell components in terms of kinetics, thermodynamics, spatio-temporal organization, NMR and X-ray structural biology, as well as single-molecule detection represent a major focus of the journal. Theoretical and computational treatments of biomacromolecular systems, macromolecular interactions, regulatory control and systems biology are also of interest to the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


