Neuroprotective effects of G9a inhibition and cannabinoid receptor activation in Alzheimer's disease through a pharmacological approach
IF 6.9
2区 医学
Q1 CLINICAL NEUROLOGY
引用次数: 0
Abstract
Epigenetic alterations are key contributors to Alzheimer's disease (AD), driving age-related cognitive decline. This study explores the combined neuroprotective effects of G9a histone methyltransferase inhibition (via UNC0642) and cannabinoid receptor activation (CB1R: ACEA; CB2R: JWH133) in AD models. We used HEK-293T cells and hippocampal neurons to demonstrate that G9a inhibition selectively enhances CB1R-mediated ERK/cAMP signaling. In SAMP8 mice (sporadic AD model), we evaluated the effects of pharmacological inhibition of G9a (UNC0642), combined with CB1R agonism (ACEA) and/or CB2R agonism (JWH133), on cognitive recovery, neuronal morphology, and neuroinflammation. Our results demonstrated that SAMP8 mice treated with UNC0642 and ACEA exhibited significant recovery in short-term memory, as assessed by the Novel Object Recognition Test (NORT), and complete recovery of spatial memory in the Object Location Test (OLT). These improvements were accompanied by enhanced neuronal morphology (increased dendritic length and density) and reduced neuroinflammation markers, suggesting a synergistic effect of G9a inhibition and CB1R activation. Importantly, JWH133 treatment, both alone and in combination with UNC0642, resulted in a pronounced reduction of neuroinflammatory markers (Trem2, Cd33, iNOS) and a significant restoration of dendritic spine density and branching length, with the dual treatment showing the most robust effects. JWH133 alone produced moderate cognitive improvement, but its combination with G9a inhibition led to outcomes comparable to those of control animals. Thus, the results underscore G9a inhibition's potential to amplify cannabinoid receptor-mediated neuroprotection while mitigating psychoactive risks, offering a promising multi-target approach for neurodegenerative diseases.
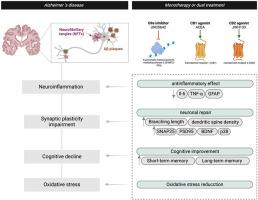
G9a抑制和大麻素受体激活在阿尔茨海默病中的神经保护作用
表观遗传改变是阿尔茨海默病(AD)的关键因素,导致与年龄相关的认知能力下降。本研究探讨了G9a组蛋白甲基转移酶抑制(通过UNC0642)和大麻素受体激活(CB1R: ACEA;CB2R: JWH133)在AD模型。我们使用HEK-293T细胞和海马神经元来证明G9a抑制选择性地增强cb1r介导的ERK/cAMP信号。在SAMP8小鼠(散发性AD模型)中,我们评估了G9a (UNC0642)联合CB1R激动剂(ACEA)和/或CB2R激动剂(JWH133)的药理抑制对认知恢复、神经元形态和神经炎症的影响。我们的研究结果表明,用UNC0642和ACEA治疗的SAMP8小鼠在新物体识别测试(NORT)中表现出短期记忆的显著恢复,在物体定位测试(OLT)中表现出空间记忆的完全恢复。这些改善伴随着神经元形态的增强(树突长度和密度增加)和神经炎症标志物的减少,表明G9a抑制和CB1R激活的协同作用。重要的是,JWH133治疗,无论是单独治疗还是与UNC0642联合治疗,都能显著降低神经炎症标志物(Trem2, Cd33, iNOS),并显著恢复树突棘密度和分支长度,双重治疗显示出最强大的效果。单独使用JWH133可产生中度认知改善,但与G9a抑制联合使用的结果与对照动物相当。因此,研究结果强调了G9a抑制在增强大麻素受体介导的神经保护的同时降低精神活性风险的潜力,为神经退行性疾病提供了一种有前途的多靶点方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neurotherapeutics
医学-神经科学
CiteScore
11.00
自引率
3.50%
发文量
154
审稿时长
6-12 weeks
期刊介绍:
Neurotherapeutics® is the journal of the American Society for Experimental Neurotherapeutics (ASENT). Each issue provides critical reviews of an important topic relating to the treatment of neurological disorders written by international authorities.
The Journal also publishes original research articles in translational neuroscience including descriptions of cutting edge therapies that cross disciplinary lines and represent important contributions to neurotherapeutics for medical practitioners and other researchers in the field.
Neurotherapeutics ® delivers a multidisciplinary perspective on the frontiers of translational neuroscience, provides perspectives on current research and practice, and covers social and ethical as well as scientific issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


