Calreticulin (crt-1) silencing reduces Aß1–42-induced toxicity and restores muscle function in C. elegans.
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2025-06-03
DOI:10.1016/j.bbadis.2025.167946
引用次数: 0
Abstract
Accumulation of aggregated β-amyloid peptide is a key histopathological feature of Alzheimer's Disease (AD). Experimental models of AD based on β-amyloid peptide display calcium (Ca2+) signaling alterations, and targeting key components of the cellular Ca2+ signaling system has been postulated to modulate AD onset and progression. Here we have taken advantage of a C. elegans strain that over-expresses the most toxic human ß-amyloid peptide (Aß1–42) in body-wall muscle cells, to study the impact of calreticulin (crt-1) silencing on body-wall muscle performance. Crt-1 knockdown reduced the percentage of paralyzed worms in a dose-dependent manner and improved locomotion parameters in free-mobility assays in Aß1–42-overexpressing worms. At the cellular level, crt-1 silencing prevented Aß1–42-induced exacerbated mitochondrial respiration and mitochondrial ROS production without impacting mitochondrial sarcomere organization. Crt-1 knockdown reduced the number and size of Aß1–42 aggregates in body-wall muscle cells and prevented the formation of Aß1–42 oligomers. We propose that crt-1 depletion reduces the number of Aß1–42 aggregates, precluding Aß1–42-induced mitochondrial toxicity and improving muscle function. We identify C. elegans crt-1 as a gene involved in the toxicity associated with the expression of human Aß1–42, and thus a potential new target for treatment.
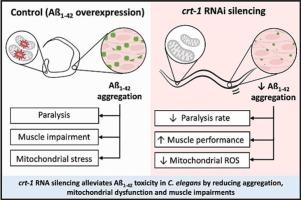
钙网蛋白(crt-1)沉默可降低a ß1 - 42诱导的毒性并恢复秀丽隐杆线虫的肌肉功能。
聚集的β-淀粉样肽的积累是阿尔茨海默病(AD)的一个关键的组织病理学特征。基于β-淀粉样肽的AD实验模型显示钙(Ca2+)信号的改变,并且靶向细胞Ca2+信号系统的关键成分被认为可以调节AD的发病和进展。在这里,我们利用秀丽隐杆线虫菌株在体壁肌肉细胞中过度表达毒性最强的人类ß-淀粉样肽(a ß1 - 42),来研究钙网蛋白(rt-1)沉默对体壁肌肉性能的影响。rt-1敲除以剂量依赖性的方式降低了a ß1 - 42过表达蠕虫的瘫痪百分比,并改善了自由移动试验中的运动参数。在细胞水平上,rt-1沉默可阻止a ß1 - 42诱导的线粒体呼吸和线粒体ROS生成加剧,但不影响线粒体肌节组织。rt-1敲低可减少体壁肌细胞中Aß1-42聚集体的数量和大小,阻止Aß1-42低聚物的形成。我们提出,crt-1耗损可减少Aß1-42聚集物的数量,从而排除Aß1-42诱导的线粒体毒性并改善肌肉功能。我们发现秀丽隐杆线虫的crt-1是一个与人类a ß1 - 42表达相关的毒性基因,因此是一个潜在的新治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


