A molecular basis of Ferredoxin Reductase (FdxR) mutations that result in mitochondriopathies
IF 3.2
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
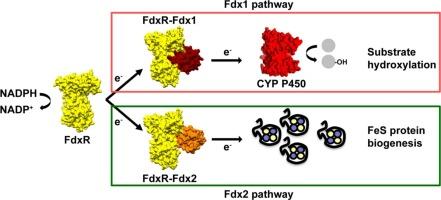
Inherited mutations in the Ferredoxin Reductase (FdxR) gene can result in a spectrum of disorders that include auditory and optic neural atrophies as well as adrenal insufficiency. FdxR (also referred to as Adrenodoxin Reductase) is a flavoprotein located in the inner mitochondrial membrane. It is responsible for mediating electron transfer from NADPH to either Fdx1 (Adrenodoxin), which is the sole reductant for all seven mitochondrial cytochromes P450, or to the related ferredoxin Fdx2, which is a component in the Fe![]() S cluster biogenesis pathway. In most cases, the mechanistic causes that underpin FdxR-related neuropathies and steroid imbalances remain unknown. In this study, we investigate three clinically relevant variants of FdxR (R211Q, R275C, and R355Q) that exhibit classic FdxR-related disease phenotypes and are widely distributed in the protein. We use a combination of biophysical and biochemical techniques to evaluate both the FdxR:Fdx1 complex and the FdxR:Fdx2 complex since these redox complexes represent an important branch point in FdxR function. Two key findings from this study are that i) all three mutants alter the recognition of Fdx1 and Fdx2, despite R275C and R355Q being located distally from the expected site of interaction, and ii) R275C and R355Q disrupt the functional complex with Fdx1, but not with Fdx2. These findings are supplemented with 2D NMR data of each mutant FdxR complex. In summary, this work implicates protein instability and degradation as the proximal cause of FdxR-related disease, with a secondary cause being the disruption of cytochrome P450-mediated metabolism in mitochondria.
S cluster biogenesis pathway. In most cases, the mechanistic causes that underpin FdxR-related neuropathies and steroid imbalances remain unknown. In this study, we investigate three clinically relevant variants of FdxR (R211Q, R275C, and R355Q) that exhibit classic FdxR-related disease phenotypes and are widely distributed in the protein. We use a combination of biophysical and biochemical techniques to evaluate both the FdxR:Fdx1 complex and the FdxR:Fdx2 complex since these redox complexes represent an important branch point in FdxR function. Two key findings from this study are that i) all three mutants alter the recognition of Fdx1 and Fdx2, despite R275C and R355Q being located distally from the expected site of interaction, and ii) R275C and R355Q disrupt the functional complex with Fdx1, but not with Fdx2. These findings are supplemented with 2D NMR data of each mutant FdxR complex. In summary, this work implicates protein instability and degradation as the proximal cause of FdxR-related disease, with a secondary cause being the disruption of cytochrome P450-mediated metabolism in mitochondria.
铁氧还蛋白还原酶(FdxR)突变导致线粒体疾病的分子基础
铁氧还蛋白还原酶(FdxR)基因的遗传突变可导致一系列疾病,包括听觉和视神经萎缩以及肾上腺功能不全。FdxR(也称为肾上腺素还氧素还原酶)是一种位于线粒体内膜的黄素蛋白。它负责介导NADPH向Fdx1(肾上腺氧还蛋白)的电子转移,Fdx1是所有七种线粒体细胞色素P450的唯一还原剂,或向相关的铁氧还蛋白Fdx2的电子转移,Fdx2是FeS簇生物发生途径的一个组成部分。在大多数情况下,支持fdxr相关神经病变和类固醇失衡的机制原因尚不清楚。在本研究中,我们研究了FdxR的三种临床相关变异(R211Q、R275C和R355Q),它们表现出经典的FdxR相关疾病表型,并广泛分布于蛋白中。我们使用生物物理和生化技术的组合来评估FdxR:Fdx1复合物和FdxR:Fdx2复合物,因为这些氧化还原复合物代表了FdxR功能的重要分支点。本研究的两个关键发现是:i)尽管R275C和R355Q位于预期的相互作用位点远端,但这三个突变体都改变了对Fdx1和Fdx2的识别;ii) R275C和R355Q破坏了Fdx1的功能复合物,而不是Fdx2。这些发现补充了每个突变FdxR复合物的二维核磁共振数据。总之,这项工作暗示蛋白质不稳定和降解是fdxr相关疾病的近端原因,其次是线粒体中细胞色素p450介导的代谢被破坏。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


