State-dependent central synaptic regulation by GLP-1 is essential for energy homeostasis
IF 20.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
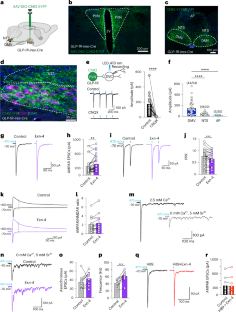
Central glucagon-like peptide-1 (GLP-1), secreted by a distinct population of nucleus tractus solitarius neurons, suppresses feeding but the exact mechanisms of action in the brain remain unclear. Here, we investigate a descending circuit formed by GLP-1 receptor (GLP-1R) neurons in the paraventricular hypothalamic nucleus (PVNGLP-1R) projecting to the dorsal vagal complex (DVC) of the brain stem in mice. PVNGLP-1R→DVC synapses release glutamate and are augmented by GLP-1. Chemogenetic activation of PVNGLP-1R→DVC suppresses feeding. Under an energy deficit (that is, hunger) state, synaptic strength is weaker but is more profoundly augmented by GLP-1R activation than under energy-replete state. In an obese condition, the dynamic synaptic changes in this circuit are disrupted. Optogenetic activation of PVNGLP-1R→DVC projections suppresses food intake energy state dependently, and blocking its synaptic release or ablating GLP-1Rs in the presynaptic neurons impairs metabolic health. These findings indicate that the state-dependent synaptic regulation by GLP-1 in PVNGLP-1R→DVC descending circuit is important for energy homeostasis. In this paper, the authors describe the energy state-dependent regulation of the PVNGLP-1R to DVC circuit, resulting in altered food intake and metabolic health, mediated by GLP-1 receptor signalling.

GLP-1的状态依赖性中枢突触调节对能量稳态至关重要
中枢胰高血糖素样肽-1 (GLP-1)由独特的孤束核神经元分泌,可抑制进食,但其在大脑中的确切作用机制尚不清楚。在这里,我们研究了由GLP-1受体(GLP-1R)神经元在室旁下丘脑核(PVNGLP-1R)中形成的投射到脑干背迷走复合体(DVC)的下行回路。PVNGLP-1R→DVC突触释放谷氨酸并被GLP-1增强。PVNGLP-1R→DVC的化学激活抑制摄食。在能量不足(即饥饿)状态下,突触强度较弱,但与能量充足状态相比,GLP-1R的激活更深刻地增强了突触强度。在肥胖的情况下,该回路的动态突触变化被破坏。光遗传激活PVNGLP-1R→DVC投射依赖性地抑制食物摄入能量状态,阻断其突触释放或消融突触前神经元中的glp - 1r会损害代谢健康。这些结果表明,GLP-1在PVNGLP-1R→DVC下行回路中的状态依赖性突触调节对能量稳态起重要作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


