Inhibition of mitochondrial oxidative stress and apoptosis in the protection of Ginkgo biloba extract 50 against cognitive impairment
IF 5.4
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Ethnopharmacological relevance
Ginkgo biloba L., a traditional medicinal plant with a long history of use in China, has been widely employed to promote blood circulation, relieve asthma, and enhance memory. It is also used in the management of cardiovascular and cerebrovascular conditions in various traditional practices. Although Ginkgo biloba shows promise in improving cognitive function and vascular health, its specific efficacy and underlying mechanism in cerebral small vessel disease remain unclear.
Aim of the study
Cerebral small vessel disease (CSVD), a leading cause of cognitive decline and dementia, currently lacks effective treatment options. As a new generation extract of Ginkgo biloba L., Ginkgo biloba extract 50 (GBE50) was developed to investigate its potential therapeutic effects on CSVD and the underlying mechanisms.
Materials and methods
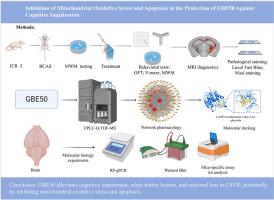
The bilateral carotid artery stenosis (BCAS) model was established in mice using microcoils, followed by therapeutic intervention. Cognitive function was assessed via the Morris water maze and Y-maze tests, while motor activity was evaluated using the open-field test. White matter and neuronal damage were analyzed through MRI, Luxol Fast Blue staining, and Nissl staining. The blood-active ingredients of GBE50 were identified using UPLC-Q-TOF-MS. Network pharmacology was applied to predict potential therapeutic targets of GBE50 against CSVD, and molecular docking was conducted to evaluate the binding interactions between key compounds and related proteins. The predicted targets and pathways were further validated through reverse transcription quantitative PCR (RT-qPCR, Western blot, and biochemical assays).
Results
GBE50 significantly improved cognitive impairment in BCAS mice. MRI and histological analyses confirmed that GBE50 alleviated white matter lesions and hippocampal neuronal loss. Twenty ingredients, including quercetin, kaempferol, and isorhamnetin, were identified in GBE50, with 80 potential therapeutic targets. Molecular docking revealed strong binding affinities between key GBE50 compounds and apoptosis-related proteins such as CASP3, CASP9, BAX, BCL-2, and Cytochrome c. Twenty key genes were also validated by RT-qPCR. BCAS induced oxidative stress, inflammation, and mitochondrial apoptosis-related gene expression, which were mitigated by GBE50. BCAS increased malondialdehyde (MDA) levels and the NADP+/NADPH ratio while decreasing superoxide dismutase (SOD), and these imbalances were reversed by GBE50. Expression of Cytochrome c, BAX, and CASP3 was elevated, whereas BCL-2 was reduced in BCAS mice, effects counteracted by GBE50.
Conclusions
GBE50 alleviates cognitive impairment, white matter lesions, and neuronal loss in CSVD, potentially by inhibiting mitochondrial oxidative stress and apoptosis.
银杏叶提取物50对线粒体氧化应激和细胞凋亡的保护作用
民族药理学相关性:银杏(Ginkgo biloba L.)是中国历史悠久的传统药用植物,具有促进血液循环、缓解哮喘、增强记忆等功效。在各种传统做法中,它也用于心脑血管疾病的管理。虽然银杏叶有改善认知功能和血管健康的前景,但其在脑血管疾病中的具体疗效和潜在机制尚不清楚。研究目的:脑血管病(CSVD)是认知能力下降和痴呆的主要原因,目前缺乏有效的治疗方案。银杏叶提取物50 (Ginkgo biloba extract 50, GBE50)作为新一代银杏叶提取物,旨在研究其对心血管疾病的潜在治疗作用及其机制。材料与方法:采用微线圈法建立小鼠双侧颈动脉狭窄(BCAS)模型,并进行治疗干预。认知功能通过Morris水迷宫和y迷宫测试进行评估,而运动活动采用开放场地测试进行评估。MRI、Luxol Fast Blue染色、Nissl染色分析脑白质及神经元损伤。采用UPLC-Q-TOF-MS对GBE50的血药活性成分进行鉴定。应用网络药理学预测GBE50治疗CSVD的潜在靶点,并进行分子对接评估关键化合物与相关蛋白的结合相互作用。通过反转录定量PCR (RT-qPCR, Western blot和生化分析)进一步验证预测的靶点和途径。结果:GBE50显著改善BCAS小鼠认知功能障碍。MRI和组织学分析证实GBE50减轻了白质病变和海马神经元损失。在GBE50中鉴定出槲皮素、山奈酚、异鼠李素等20种成分,具有80个潜在的治疗靶点。分子对接发现,GBE50关键化合物与凋亡相关蛋白CASP3、CASP9、BAX、BCL-2、Cytochrome c等具有较强的结合亲和力。20个关键基因也通过RT-qPCR验证。BCAS诱导氧化应激、炎症和线粒体凋亡相关基因表达,GBE50可减轻这些基因表达。BCAS增加丙二醛(MDA)水平和NADP+/NADPH比值,同时降低超氧化物歧化酶(SOD),这些失衡被GBE50逆转。细胞色素c、BAX和CASP3的表达升高,而BCL-2在BCAS小鼠中表达降低,这种作用被GBE50抵消。结论:GBE50可能通过抑制线粒体氧化应激和细胞凋亡,减轻CSVD患者的认知功能障碍、白质病变和神经元丢失。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of ethnopharmacology
医学-全科医学与补充医学
CiteScore
10.30
自引率
5.60%
发文量
967
审稿时长
77 days
期刊介绍:
The Journal of Ethnopharmacology is dedicated to the exchange of information and understandings about people''s use of plants, fungi, animals, microorganisms and minerals and their biological and pharmacological effects based on the principles established through international conventions. Early people confronted with illness and disease, discovered a wealth of useful therapeutic agents in the plant and animal kingdoms. The empirical knowledge of these medicinal substances and their toxic potential was passed on by oral tradition and sometimes recorded in herbals and other texts on materia medica. Many valuable drugs of today (e.g., atropine, ephedrine, tubocurarine, digoxin, reserpine) came into use through the study of indigenous remedies. Chemists continue to use plant-derived drugs (e.g., morphine, taxol, physostigmine, quinidine, emetine) as prototypes in their attempts to develop more effective and less toxic medicinals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


