Identifying antimalarials that disrupt malaria parasite transmission when fed to the mosquito
IF 3.2
2区 医学
Q1 PARASITOLOGY
引用次数: 0
Abstract
A decade-long decline in malaria cases has plateaued, primarily due to parasite drug resistance and mosquito resistance to insecticides used in bed nets and indoor residual spraying. Here, we explore the innovative control strategy targeting Plasmodium with antimalarials during the mosquito stages. This strategy has the potential to reduce the risk of resistance emerging because a relatively small population of parasites within the mosquito is subject to selection. After validating mosquito feeding strategies, we screened a range of parasiticidal compounds by feeding them to mosquitoes already infected with mouse malaria (P. berghei). Three antimalarials showed activity against P. berghei in mosquitoes, apparently targeting specific stages of P. berghei development during transmission. Borrelidin, a threonyl-tRNA synthetase inhibitor, significantly reduced P. berghei sporozoite numbers. Azithromycin, an antibiotic targeting apicoplast protein synthesis, significantly lowered sporozoite infectivity in mice. T111, a next generation compound targeting the parasite electron transport chain, reduced sporozoite numbers in P. berghei at equivalent concentrations to the gold standard electron transport chain inhibitor, atovaquone. T111 also prevented sporozoite production in mosquitoes infected with human malaria, P. falciparum, even after very short exposure times. Encouragingly, T111 remained efficacious after being freeze-dried onto a substrate and later reconstituted with water, suggesting this compound would be effective in easy-to-distribute-and-deploy transmission control devices. Our findings suggest that several antimalarials can be used to target mosquito-stage parasites via sugar baits and limit malaria transmission. Importantly, mosquito feeding of antimalarials could vastly increase the range of potentially useful parasiticidal compounds to include those failing to meet the exacting standards required for human antimalarial drugs, potentially improving malaria control for minimal cost.
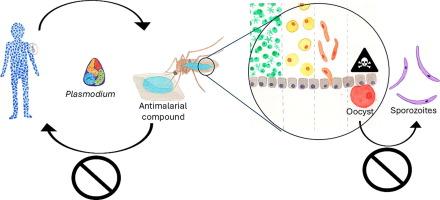
确定喂给蚊子的抗疟疾药物可阻断疟疾寄生虫的传播。
疟疾病例十年来的下降势头已趋于稳定,这主要是由于寄生虫对药物产生耐药性,以及蚊子对蚊帐和室内残留喷洒杀虫剂产生抗药性。在此,我们探索了在蚊子阶段使用抗疟药物靶向疟原虫的创新控制策略。这一策略有可能降低出现耐药性的风险,因为蚊子体内相对较小的寄生虫种群受到选择的影响。在验证了蚊子的取食策略后,我们通过将这些化合物喂给已经感染了小鼠疟疾(P. berghei)的蚊子来筛选一系列杀寄生虫化合物。三种抗疟药在蚊子中显示出对伯氏疟原虫的活性,显然是针对伯氏疟原虫传播过程中的特定发展阶段。苏酰trna合成酶抑制剂Borrelidin可显著降低柏氏假体孢子体的数量。阿奇霉素是一种靶向顶质体蛋白合成的抗生素,可显著降低小鼠孢子体的感染性。T111是一种靶向寄生虫电子传递链的新一代化合物,在与金标准电子传递链抑制剂阿托伐醌相当的浓度下,可以减少柏氏假体的孢子体数量。T111还能防止感染人类疟疾(恶性疟原虫)的蚊子产生孢子,即使接触时间很短。令人鼓舞的是,T111在基材上冷冻干燥后仍然有效,随后用水重组,这表明该化合物在易于分布和部署的传动控制装置中有效。我们的研究结果表明,几种抗疟疾药物可以通过糖诱饵靶向蚊子阶段的寄生虫,并限制疟疾的传播。重要的是,以蚊子为食的抗疟疾药物可以大大增加潜在有用的杀寄生虫化合物的范围,包括那些不符合人类抗疟疾药物所需的严格标准的化合物,从而可能以最低的成本改善疟疾控制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.40
自引率
2.50%
发文量
76
审稿时长
23 days
期刊介绍:
International Journal for Parasitology offers authors the option to sponsor nonsubscriber access to their articles on Elsevier electronic publishing platforms. For more information please view our Sponsored Articles page. The International Journal for Parasitology publishes the results of original research in all aspects of basic and applied parasitology, including all the fields covered by its Specialist Editors, and ranging from parasites and host-parasite relationships of intrinsic biological interest to those of social and economic importance in human and veterinary medicine and agriculture.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


