Microglia-drive IRF8 upregulates complement pathway in Parkinson's disease
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2025-05-31
DOI:10.1016/j.bbadis.2025.167928
引用次数: 0
Abstract
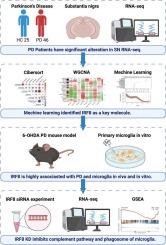
Parkinson's disease (PD) is a widespread degenerative disorder of the central nervous system. The gradual degeneration of dopaminergic neurons in the substantia nigra region is one of the primary pathological features of PD. Glial cells in SN are also linked to the pathological PD alterations. To discern the role of neurons and glial cells as well as their corresponding genetic modifications in PD, we utilized diverse bioinformatics techniques and performed biological experiments on cell and animal models. Several transcriptome datasets of the substantia nigra region were collected from the Gene Expression Omnibus dataset. Cibersort was used to deconvolute the data into proportions of brain cell types. WGCNA was used to analyze the association between modules and traits. Machine learning was used to select the hub genes from WGCNA results. Based on the results of transcriptome analysis, microglia were the most related cell type. Through machine learning, IRF8 was identified as the hub gene associated with PD and microglia. Furthermore, an increased ratio of IRF8+ microglia was observed in PD mice, along with an elevated expression of IRF8 in primary microglia cultures treated with α-synuclein preformed fibril (PFFs). To explore the function of IRF8 in microglia under disease condition, we conducted siRNA of IRF8 and found it was highly associated with complement pathway, which may cause the activation of microglia. In conclusion, our research indicated IRF8 may be involved in the functional regulation of microglia in PD.
小胶质细胞驱动的IRF8上调帕金森病补体通路
帕金森病(PD)是一种广泛的中枢神经系统退行性疾病。黑质区多巴胺能神经元的逐渐变性是帕金森病的主要病理特征之一。SN中的神经胶质细胞也与PD的病理改变有关。为了了解神经元和神经胶质细胞在PD中的作用及其相应的遗传修饰,我们利用多种生物信息学技术,在细胞和动物模型上进行了生物学实验。从Gene Expression Omnibus数据集中收集了几个黑质区域的转录组数据集。使用Cibersort将数据反卷积成脑细胞类型的比例。利用WGCNA分析模块与性状之间的关联关系。利用机器学习从WGCNA结果中选择中心基因。根据转录组分析结果,小胶质细胞是最相关的细胞类型。通过机器学习,IRF8被确定为PD和小胶质细胞相关的枢纽基因。此外,在PD小鼠中观察到IRF8+小胶质细胞的比例增加,α-突触核蛋白预形成纤维(PFFs)处理的原代小胶质细胞中IRF8的表达升高。为了探究疾病状态下IRF8在小胶质细胞中的功能,我们对IRF8进行了siRNA检测,发现其与补体通路高度相关,可能导致小胶质细胞活化。综上所述,我们的研究提示IRF8可能参与PD小胶质细胞的功能调控。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


