MitoQ for vitiligo by mitigating PARP1 translocation aberrations: Network pharmacology and experimental validation
IF 3.7
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular cell research
Pub Date : 2025-05-30
DOI:10.1016/j.bbamcr.2025.119995
引用次数: 0
Abstract
Purpose
Oxidative stress plays a significant role in the development of vitiligo. Although the specific mechanism of the mitochondria-targeted antioxidant mitoquinone (MitoQ) in vitiligo remains unclear, it has shown promise in the treatment of various diseases.
Methods
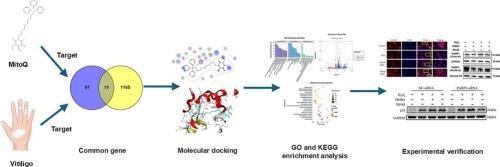
In this study, we employed network pharmacology, molecular docking, transcriptomic approaches, and experimental verification to investigate the potential targets of MitoQ in vitiligo.
Results
Molecular docking results identified four possible crucial targets of MitoQ in vitiligo treatment: poly (ADP-ribose) polymerase 1 (PARP1), prostaglandin-endoperoxide synthase 2 (PTGS2), estrogen receptor 1 (ESR1), and C-X-C motif chemokine receptor 3 (CXCR3). MitoQ alleviated oxidative stress-induced PARP1 nuclear mislocalization, attenuated ROS accumulation, restored mitochondrial membrane potential, and enhanced ATP synthesis in vitro analysis. Transcriptomic analysis demonstrated that MitoQ reduced the expression of DNA damage genes and genes involved in the PI3K-AKT and MAPK signaling pathways. The protein-protein interaction network indicated a potential relationship between PARP1 and DNA damage-related genes, suggesting that MitoQ could interfere with abnormal PARP1 activation. Notably, MitoQ reduced cellular senescence by decreasing CDKN1A/p21 protein through PARP1, and the knockdown of PARP1 reduced oxidative damage.
Conclusion
These results indicate that PARP1 decreases cellular senescence and offers a potential target for therapeutic research in the management of vitiligo.
MitoQ通过减轻PARP1易位畸变治疗白癜风:网络药理学和实验验证
目的氧化应激在白癜风发病过程中起重要作用。虽然线粒体靶向抗氧化剂mito醌(MitoQ)在白癜风中的具体机制尚不清楚,但它在多种疾病的治疗中显示出前景。方法采用网络药理学、分子对接、转录组学、实验验证等方法研究MitoQ在白癜风中的潜在靶点。结果分子对接结果确定了MitoQ在白癜风治疗中的四个可能的关键靶点:聚(adp -核糖)聚合酶1 (PARP1)、前列腺素内过氧化物合酶2 (PTGS2)、雌激素受体1 (ESR1)和C-X-C基序趋化因子受体3 (CXCR3)。体外分析表明,MitoQ可减轻氧化应激诱导的PARP1核错定位,减少ROS积累,恢复线粒体膜电位,增强ATP合成。转录组学分析表明,MitoQ降低了DNA损伤基因以及参与PI3K-AKT和MAPK信号通路的基因的表达。蛋白-蛋白相互作用网络表明PARP1与DNA损伤相关基因之间存在潜在关系,表明MitoQ可能干扰PARP1的异常激活。值得注意的是,MitoQ通过PARP1降低CDKN1A/p21蛋白,从而减少细胞衰老,而PARP1的敲低降低了氧化损伤。结论PARP1可延缓白癜风细胞衰老,为白癜风治疗研究提供了潜在靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.00
自引率
2.00%
发文量
151
审稿时长
44 days
期刊介绍:
BBA Molecular Cell Research focuses on understanding the mechanisms of cellular processes at the molecular level. These include aspects of cellular signaling, signal transduction, cell cycle, apoptosis, intracellular trafficking, secretory and endocytic pathways, biogenesis of cell organelles, cytoskeletal structures, cellular interactions, cell/tissue differentiation and cellular enzymology. Also included are studies at the interface between Cell Biology and Biophysics which apply for example novel imaging methods for characterizing cellular processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


