Improving neuronal recovery in spinal cord injury with NEP1-40-modified neural stem cells through RhoA/ROCK signaling pathway modulation
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2025-05-28
DOI:10.1016/j.bbadis.2025.167929
引用次数: 0
Abstract
To evaluate the therapeutic potential of NEP1-40-expressing neural stem cells (NSCs) in improving outcomes in spinal cord injury (SCI) by modulating the RhoA/ROCK signaling pathway. NEP1-40 was overexpressed by transfecting isolated NSCs with NEP1-40 lentivirus, and their differentiation following Nogo-A treatment was assessed via Tuj-1 immunofluorescence. The effects of NEP1-40 overexpression on axonal damage of differentiated NSCs and DRG neurons were examined using Tuj-1 and F-actin immunofluorescence, along with Western blotting for GAP-43, MAP-2, and APP. The activation of RhoA/ROCK and its downstream pathways, namely, PTEN/PI3K/AKT and MLC/LIMK, were analyzed by Western blotting. In a rat SCI model, following NSC transplantation, was assessed by examining neuronal regeneration markers. Furthermore, the Basso, Beattie, and Bresnahan (BBB) scale behavioral tests were used to assess motor function recovery. NEP1-40 overexpression inhibited the RhoA/ROCK signaling, followed by the stimulating the PI3K/AKT pathway and promoting neuronal differentiation under Nogo-A treatment. The inhibition of RhoA/ROCK signaling suppressed the MLC/LIMK pathway to enhance neurite outgrowth in NSC-differentiated and DRG neurons. In SCI rats, NEP1-40 overexpression increased the number of NSCs and neurons, whereas NEP1-40 overexpression in NSCs significantly increased the number of 5-HT-, ChAT-, and CGRP-positive neurons, as well as the levels of p-GAP-43, GAP-43 and MAP-2, and reduced the level of APP. These changes were accompanied by improved BBB scores. NEP1-40-modified NSCs enhance SCI recovery by activating the PI3K/AKT pathway and inhibiting the MLC/LIMK cascade, thereby promoting neuronal differentiation and reducing axonal damage. Altogether, this approach demonstrates promising therapeutic potential to improve NSC efficacy in SCI.
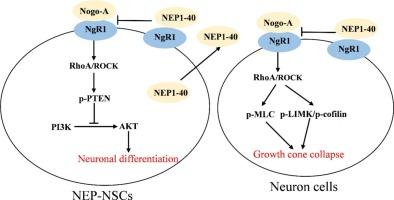
通过RhoA/ROCK信号通路调节nep1 -40修饰的神经干细胞促进脊髓损伤神经元恢复
评价表达nep1 -40的神经干细胞(NSCs)通过调节RhoA/ROCK信号通路改善脊髓损伤(SCI)预后的治疗潜力。用NEP1-40慢病毒转染分离的NSCs后,NEP1-40过表达,通过Tuj-1免疫荧光检测Nogo-A治疗后NEP1-40的分化情况。采用Tuj-1和F-actin免疫荧光检测NEP1-40过表达对分化的NSCs和DRG神经元轴突损伤的影响,并结合GAP-43、MAP-2和APP的Western blot检测。Western blot检测RhoA/ROCK及其下游通路PTEN/PI3K/AKT和MLC/LIMK的激活情况。在大鼠脊髓损伤模型中,NSC移植后,通过检测神经元再生标志物进行评估。此外,采用Basso, Beattie, and Bresnahan (BBB)量表行为测试评估运动功能恢复。在Nogo-A处理下,NEP1-40过表达抑制RhoA/ROCK信号通路,进而刺激PI3K/AKT通路,促进神经元分化。RhoA/ROCK信号的抑制抑制了MLC/LIMK通路,促进了nsc分化和DRG神经元的神经突生长。在脊髓损伤大鼠中,NEP1-40过表达增加了NSCs和神经元的数量,而NSCs中NEP1-40过表达显著增加了5-HT、ChAT-和cgrp阳性神经元的数量,以及p-GAP-43、GAP-43和MAP-2的水平,并降低了APP的水平。这些变化伴随着BBB评分的提高。nep1 -40修饰的NSCs通过激活PI3K/AKT通路,抑制MLC/LIMK级联,从而促进神经元分化,减轻轴突损伤,从而促进脊髓损伤的恢复。总之,这种方法在提高NSC对脊髓损伤的疗效方面显示出良好的治疗潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


