Hydrogen inhalation and intrathecal magnesium sulfate ameliorate ischemia by suppressing cortical spreading depolarization in a rat subarachnoid hemorrhage model
IF 6.9
2区 医学
Q1 CLINICAL NEUROLOGY
引用次数: 0
Abstract
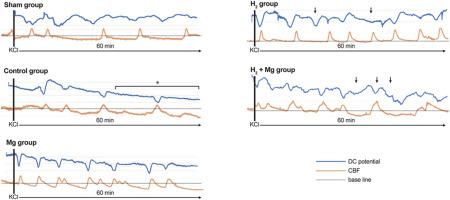
This study investigated whether inhaled hydrogen and intrathecal magnesium could mitigate cortical spreading depolarization and delayed cerebral ischemia in a rat model of subarachnoid hemorrhage. Adult male rats underwent subarachnoid hemorrhage induction with nitric oxide synthase inhibition and high-potassium application to elicit cortical spreading depolarization. Animals were assigned to sham, control, H2, Mg, or combined H2 and Mg treatment groups. We measured direct current potentials, cerebral blood flow, brain water content, bodyweight changes, and neurological outcomes. Compared with controls, the H2 and Mg groups had significantly reduced total depolarization and hypoperfusion times. The combined treatment produced similar benefits. H2 alone rapidly shortened depolarization duration, suggesting that it may offer neuroprotection until Mg effects fully manifest. Neither treatment altered physiological parameters, brain water content, bodyweight, or neurological deficits. These findings indicate that H2 and Mg reduce key pathophysiological processes related to early brain injury and delayed cerebral ischemia following subarachnoid hemorrhage, potentially improving outcomes by minimizing depolarization events and associated ischemia. H2 therapy may provide early protective effects before Mg exertion.
氢吸入和鞘内硫酸镁通过抑制大鼠蛛网膜下腔出血模型皮质扩张性去极化改善缺血。
本研究探讨了吸入氢和鞘内镁是否能减轻大鼠蛛网膜下腔出血模型皮质扩张性去极化和延迟性脑缺血。采用一氧化氮合酶抑制和高钾诱导成年雄性大鼠蛛网膜下腔出血诱导皮层扩张性去极化。动物被分为假药、对照组、H2、Mg或H2和Mg联合治疗组。我们测量了直流电电位、脑血流量、脑含水量、体重变化和神经预后。与对照组相比,H2组和Mg组的总去极化和低灌注时间显著减少。联合治疗也产生了类似的效果。单独H2可迅速缩短去极化持续时间,表明它可能在Mg效应完全显现之前提供神经保护。两种治疗方法都没有改变生理参数、脑含水量、体重或神经功能缺陷。这些发现表明,H2和Mg可以减少蛛网膜下腔出血后早期脑损伤和延迟性脑缺血相关的关键病理生理过程,可能通过减少去极化事件和相关缺血来改善预后。H2治疗可在Mg作用前提供早期保护作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neurotherapeutics
医学-神经科学
CiteScore
11.00
自引率
3.50%
发文量
154
审稿时长
6-12 weeks
期刊介绍:
Neurotherapeutics® is the journal of the American Society for Experimental Neurotherapeutics (ASENT). Each issue provides critical reviews of an important topic relating to the treatment of neurological disorders written by international authorities.
The Journal also publishes original research articles in translational neuroscience including descriptions of cutting edge therapies that cross disciplinary lines and represent important contributions to neurotherapeutics for medical practitioners and other researchers in the field.
Neurotherapeutics ® delivers a multidisciplinary perspective on the frontiers of translational neuroscience, provides perspectives on current research and practice, and covers social and ethical as well as scientific issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


