Open-Label Randomized Controlled Trial and Feasibility Study of an Oral Probiotic Intervention to Reduce Group B Streptococcus Colonization in Pregnant People by the Time of Birth
Abstract
Introduction
The purpose of this midwife-led study was to determine the feasibility of a randomized controlled trial (RCT) of probiotics to reduce group B Streptococcus (GBS) colonization by the time of birth in healthy, GBS-positive, pregnant adults.
Methods
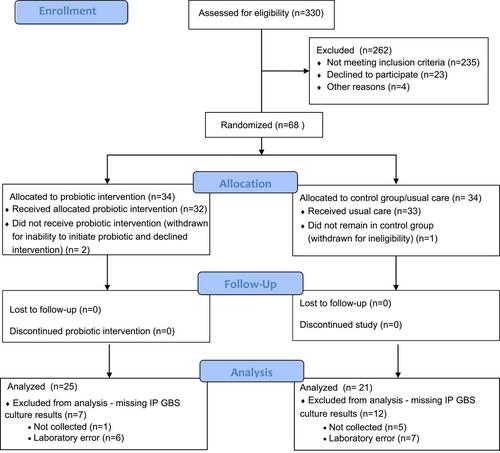
An open-label randomized clinical trial comparing Florajen Digestion, a commercially available combination oral probiotic, with usual care (ClinicalTrials.gov NCT04721912) was conducted in a midwifery practice serving a racially and ethnically diverse population. Eligible patients who tested positive for GBS at routine third-trimester screening were offered informed consent and participation. The primary outcome was feasibility for a larger RCT, including feasibility of probiotic use among participants. Secondary outcomes were intrapartum GBS colonization and Antepartum Gastrointestinal Symptoms of Pregnancy (AP-GI-SA) scores.
Results
A total of 68 participants were enrolled and randomized; 65 participants completed the study, but only 46 had intrapartum cultures collected and processed. Among the 23 pregnant individuals who were eligible but chose not to participate, 3 indicated that they did not want to take a probiotic. After an average of 14 days of the intervention, 7 of 25 (28%) participants in the probiotic group had a negative intrapartum GBS result compared with 3 of 21 (14.3%) in the control group (odds ratio, 2.33; 95% CI, 0.52-10.48). There was no difference in perinatal outcomes or AP-GI-SA scores between groups. No adverse events occurred.
Discussion
The feasibility of a larger RCT was demonstrated. Challenges identified included intrapartum GBS collection and laboratory processing during the COVID-19 pandemic. The study was not powered to detect a significant difference in intrapartum GBS colonization, although a larger decrease in GBS colonization was noted among probiotic-using participants. Florajen Digestion may show efficacy in a RCT with a longer intervention period. It is possible that the probiotic intervention duration was too brief to show a reduction in gastrointestinal pregnancy symptoms.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


