Development of a workflow for in vitro on– and off-target cytotoxicity assessment of CAR T cell therapies to support first-in-human clinical trials: An orthogonal approach using human induced pluripotent stem cell-derived cells as a surrogate for normal vital organ systems
IF 2.9
Q2 TOXICOLOGY
引用次数: 0
Abstract
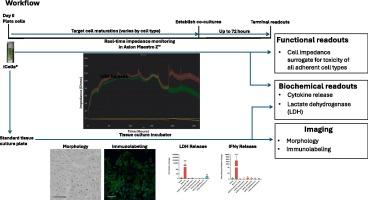
Chimeric antigen receptor (CAR) T cell therapy has revolutionized cancer therapy and is showing promise for other non-cancer disease indications. CAR T cells are directed to a target antigen that is overexpressed by tumor cells. On-target toxicity can arise if the targeted protein is also expressed in normal cell populations. Additionally, off-target toxicity may occur if the CAR T cell recognizes or cross-reacts with a non-target protein thereby activating the T cells leading to subsequent adverse sequela. Given the nature of this biological class as a human cellular product, standard safety assessments in animals are largely not appropriate as the drug product is derived from human cells and the associated CAR often lacks reactivity with the animal homolog of the target protein. Undesired targeting of healthy/normal tissue that express the intended target antigen (on-target/off-tumor), as well as unintended targeting of other antigens expressed on healthy/normal tissue is a safety concern that may be explored using human induced pluripotent stem cell (hiPSC)-derived test systems as surrogates for various normal cell types. The panel of hiPSC-derived cells is intended to broadly represent different human cell types from vital organs that can be targets for severe adverse events. Herein the development of an orthogonal approach to an in vitro co-culture assay used to assess unintended CAR T cell cytotoxicity in normal cells is described. Experimental considerations including assay and cell model qualification are presented, and an orthogonal workflow described. Finally, an illustrative case of experimental CD33 CAR T cells co-cultured with a select panel of hiPSC-derived normal cells serves as a springboard for other CAR T cell developers to consider in their nonclinical safety programs.
开发CAR - T细胞疗法的体外靶向和脱靶细胞毒性评估工作流程,以支持首次人体临床试验:使用人类诱导多能干细胞衍生细胞作为正常重要器官系统的替代品的正交方法
嵌合抗原受体(CAR) T细胞疗法已经彻底改变了癌症治疗,并在其他非癌症疾病适应症中显示出希望。CAR - T细胞被导向肿瘤细胞过度表达的靶抗原。如果靶蛋白也在正常细胞群中表达,则可能产生靶毒性。此外,如果CAR - T细胞识别非靶蛋白或与非靶蛋白发生交叉反应,从而激活T细胞,导致随后的不良后遗症,则可能发生脱靶毒性。考虑到这类生物作为人类细胞产品的性质,动物的标准安全性评估在很大程度上是不合适的,因为药物产品来源于人类细胞,相关的CAR通常与靶蛋白的动物同源物缺乏反应性。对表达预期靶抗原的健康/正常组织(靶上/肿瘤外)的意外靶向,以及对健康/正常组织上表达的其他抗原的意外靶向,是一个安全问题,可以使用人类诱导多能干细胞(hiPSC)衍生的测试系统作为各种正常细胞类型的替代品进行探索。hipsc衍生细胞组旨在广泛代表来自重要器官的不同人类细胞类型,这些细胞可能是严重不良事件的靶标。本文描述了一种用于评估正常细胞中意外CAR - T细胞毒性的体外共培养试验的正交方法的发展。实验考虑包括分析和细胞模型鉴定,并描述了正交工作流程。最后,一个实验性CD33 CAR - T细胞与hipsc衍生的正常细胞共培养的示例,为其他CAR - T细胞开发人员在其非临床安全性计划中考虑提供了一个跳板。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Current Research in Toxicology
Environmental Science-Health, Toxicology and Mutagenesis
CiteScore
4.70
自引率
3.00%
发文量
33
审稿时长
82 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


