Chemically Engineered Affinity Protein Drugs for Covalent Targeted Cancer Therapy
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
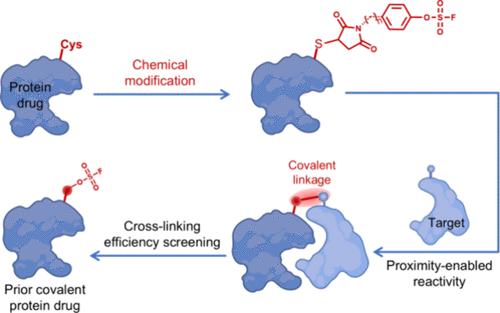
Affinity proteins are multiple types of well-explored small scaffold proteins with excellent tumor targeting performance. However, due to their small size, the balance between rapid blood clearance and efficient tumor accumulation remains a challenge for their clinical application. The covalent targeting mode, endowing the affinity proteins with an irreversible binding ability to their receptor and then decoupling the pharmacodynamic effect from pharmacokinetics, may provide a promising solution for clinical applications of affinity proteins. Herein, we develop a chemical modification strategy to construct covalently targeted affinity protein drugs. Through the chemical attachment with a sulfur(VI) fluoride exchange (SuFEx) chemistry-based maleimide-substituted aryl fluorosulfate (MFS) linker, the engineered affinity protein acquires the capacity to covalently link with its targeting receptor. As a proof of concept, the MFS linker modified affibody-protein drug elicited over 72% covalent binding to the target human epidermal growth factor receptor 2 (HER2) and 185% higher cell uptake than that of the noncovalent control in vitro. In mice, the tumor retention capacity of the covalent affibody-protein drug was 2.01 times greater than that of the control group, ultimately resulting in nearly complete inhibition of tumor growth. Similar enhanced therapeutic efficacy was also obtained in another MFS linker-armed monobody-protein drug targeting the epidermal growth factor receptor (EGFR). In brief, this facile chemical modification strategy provides a general platform for preparing covalently targeted affinity protein drugs, potentially accelerating the application of protein therapeutics in diverse diseases.
共价靶向癌症治疗的化学工程亲和蛋白药物
亲和蛋白是多种类型的小支架蛋白,具有良好的肿瘤靶向性能。然而,由于其体积小,在快速血液清除和有效肿瘤积累之间的平衡仍然是其临床应用的一个挑战。共价靶向模式赋予亲和蛋白与受体的不可逆结合能力,从而将药效学效应与药代动力学解耦,为亲和蛋白的临床应用提供了一种很有前景的解决方案。在此,我们开发了一种化学修饰策略来构建共价靶向亲和蛋白药物。通过与基于硫(VI)氟交换(SuFEx)化学的马来酰亚胺取代氟磺酸芳基(MFS)连接剂的化学附着,工程亲和蛋白获得了与其靶受体共价连接的能力。作为概念的证明,在体外,MFS连接子修饰的修饰蛋白药物与靶人表皮生长因子受体2 (HER2)的共价结合超过72%,细胞摄取比非共价对照高185%。在小鼠体内,该共价药物的肿瘤保留能力是对照组的2.01倍,最终几乎完全抑制了肿瘤的生长。另一种靶向表皮生长因子受体(EGFR)的MFS连接臂单蛋白药物也获得了类似的增强治疗效果。简而言之,这种简单的化学修饰策略为制备共价靶向亲和蛋白药物提供了一个通用平台,有可能加速蛋白质治疗在各种疾病中的应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


