Genetic depletion of adipose-derived Isthmin-1 causes hepatic steatosis
IF 6.6
2区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
Objectives
Adipose tissue plays a critical role in obesity, as its dysfunction can impair lipid homeostasis and result in lipid overflow and ectopic lipid deposition in the liver. We previously demonstrated that Isthmin-1 (Ism1) regulates glucose uptake into the adipose tissue and suppresses hepatic steatosis, but the role of adipose-derived Ism1 is unknown. Here, we investigate the role of adipose-derived Ism1 in metabolic health and its impact on hepatic steatosis and lipid metabolism.
Methods
In this study, we employed both a genetic knockout approach, selectively deleting Ism1 in adipose tissue of mice (AdipoQ-Ism1-KO), and a pharmacological approach by administering recombinant Ism1 protein to mice. These mice were subjected to a high fat-high fructose diet to simulate conditions that promote Metabolic-dysfunction Associated Steatotic Liver Disease (MASLD).
Results
AdipoQ-Ism1-KO are of normal weight, but prone to severe hepatic steatosis in response to high fat-high fructose feeding. Lipidomic profiling through untargeted analyses in both gain-of-function and loss-of-function models was used to assess changes in hepatic lipid homeostasis. These results provide in vivo genetic support for the role of Ism1 as a regulator of the adipose–hepatic axis.
Conclusions
Collectively, these data demonstrate that loss of adipose-derived Ism1 disrupts lipid homeostasis and accelerates the development of hepatic steatosis. This study provides a genetic basis for Ism1's involvement in metabolic regulation, suggesting a potential therapeutic target for treating metabolic disorders.
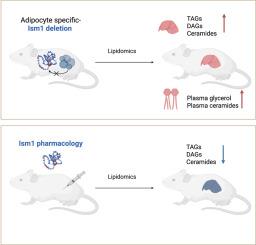
脂肪来源的Isthmin-1基因缺失导致肝脏脂肪变性。
目的:脂肪组织在肥胖中起着至关重要的作用,其功能障碍可破坏肝脏脂质稳态,导致肝脏脂质溢出和异位脂质沉积。我们之前证明Isthmin-1 (Ism1)调节葡萄糖摄取到脂肪组织并抑制肝脂肪变性,但脂肪来源的Ism1的作用尚不清楚。在这里,我们研究了脂肪来源的Ism1在代谢健康中的作用及其对肝脏脂肪变性和脂质代谢的影响。方法:在本研究中,我们采用了基因敲除方法,选择性地删除小鼠脂肪组织中的Ism1 (AdipoQ-Ism1-KO),以及药理学方法,通过给小鼠注射重组Ism1蛋白。这些小鼠接受高脂肪-高果糖饮食,以模拟促进代谢功能障碍相关脂肪变性肝病(MASLD)的条件。结果:AdipoQ-Ism1-KO体重正常,但在高脂高果糖喂养下容易发生严重的肝脏脂肪变性。通过对功能获得和功能丧失模型的非靶向分析,脂质组学分析被用于评估肝脂质稳态的变化。这些结果为Ism1作为脂肪-肝轴调节因子的作用提供了体内遗传支持。结论:总的来说,这些数据表明,脂肪源性Ism1的缺失破坏了脂质稳态,加速了肝脂肪变性的发展。本研究为Ism1参与代谢调节提供了遗传学基础,提示了治疗代谢紊乱的潜在治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
14.50
自引率
2.50%
发文量
219
审稿时长
43 days
期刊介绍:
Molecular Metabolism is a leading journal dedicated to sharing groundbreaking discoveries in the field of energy homeostasis and the underlying factors of metabolic disorders. These disorders include obesity, diabetes, cardiovascular disease, and cancer. Our journal focuses on publishing research driven by hypotheses and conducted to the highest standards, aiming to provide a mechanistic understanding of energy homeostasis-related behavior, physiology, and dysfunction.
We promote interdisciplinary science, covering a broad range of approaches from molecules to humans throughout the lifespan. Our goal is to contribute to transformative research in metabolism, which has the potential to revolutionize the field. By enabling progress in the prognosis, prevention, and ultimately the cure of metabolic disorders and their long-term complications, our journal seeks to better the future of health and well-being.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


