Phase equilibria of the system Isopropanol+Water+Isopropyl Myristate+Myristic acid
IF 2.7
3区 工程技术
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
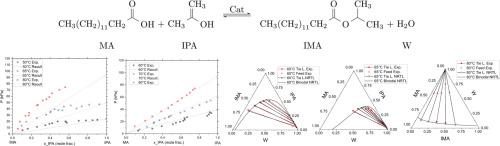
Isopropyl myristate (IMA) is a fatty ester widely used in cosmetic applications, and it is mainly produced by esterification of myristic acid (MA) and isopropanol (IPA), with water (W) as byproduct. Due to chemical equilibrium limitations, various process intensification approaches have been proposed to improve its production; however, they still involve significant uncertainties as they are based on theoretically predicted phase equilibria due to a lack of experimental-based models. Then, the presented study aimed to experimentally characterize the phase equilibrium behavior of mixtures containing IPA, W, IMA, and MA. Vapor-Liquid Equilibrium (VLE) experiments were conducted under isothermal conditions for the binary systems IMA+IPA and MA+IPA within the range of 55–80 °C. Also, Liquid-Liquid Equilibrium (LLE) data for the ternary systems IPA+W+IMA, IPA+W+MA, and W+IMA+MA were measured at temperatures ranging from 60 to 80 °C. Additionally, Solid-Liquid Equilibrium (SLE) data for mixtures of IMA+MA were obtained at temperature in-between 8 and 50 °C, and pure thermal properties of IMA and MA were characterized via Differential Scanning Calorimetry (DSC) and Thermogravimetric analysis (TGA). Using the collected VLE, LLE and SLE experimental data, binary interaction parameters for NRTL model were fitted through optimization. Conflicting requirements in VLE and LLE data were addressed through Pareto front analysis, yielding two new parameters sets: one for predominant VLE description and the other for more accurate LLE representation. These sets of parameters would be suitable for the conceptual design and simulation of sequential and/or simultaneous reaction-separation processes to produce IMA via esterification.
异丙醇+水+肉豆蔻酸异丙酯+肉豆蔻酸体系的相平衡
肉豆蔻酸异丙酯(IMA)是一种广泛应用于化妆品的脂肪酯,主要由肉豆蔻酸(MA)和异丙醇(IPA)酯化而成,副产物为水(W)。由于化学平衡的限制,人们提出了各种工艺强化方法来提高其产量;然而,由于缺乏基于实验的模型,它们仍然涉及重大的不确定性,因为它们是基于理论预测的相平衡。然后,本研究旨在通过实验表征含有IPA、W、IMA和MA的混合物的相平衡行为。在55 ~ 80℃的等温条件下,对IMA+IPA和MA+IPA二元体系进行了气液平衡(VLE)实验。此外,在60 ~ 80℃的温度范围内测量了IPA+W+IMA、IPA+W+MA和W+IMA+MA三元体系的液液平衡(LLE)数据。此外,IMA+MA混合物在8 ~ 50℃温度下的固液平衡(SLE)数据,并通过差示扫描量热法(DSC)和热重分析(TGA)对IMA和MA的纯热性质进行了表征。利用收集的VLE、LLE和SLE实验数据,优化拟合了NRTL模型的二元相互作用参数。通过帕累托前沿分析,解决了VLE和LLE数据中相互冲突的需求,产生了两个新的参数集:一个用于主要的VLE描述,另一个用于更准确的LLE表示。这些参数集将适用于通过酯化生产IMA的顺序和/或同时反应分离过程的概念设计和模拟。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fluid Phase Equilibria
工程技术-工程:化工
CiteScore
5.30
自引率
15.40%
发文量
223
审稿时长
53 days
期刊介绍:
Fluid Phase Equilibria publishes high-quality papers dealing with experimental, theoretical, and applied research related to equilibrium and transport properties of fluids, solids, and interfaces. Subjects of interest include physical/phase and chemical equilibria; equilibrium and nonequilibrium thermophysical properties; fundamental thermodynamic relations; and stability. The systems central to the journal include pure substances and mixtures of organic and inorganic materials, including polymers, biochemicals, and surfactants with sufficient characterization of composition and purity for the results to be reproduced. Alloys are of interest only when thermodynamic studies are included, purely material studies will not be considered. In all cases, authors are expected to provide physical or chemical interpretations of the results.
Experimental research can include measurements under all conditions of temperature, pressure, and composition, including critical and supercritical. Measurements are to be associated with systems and conditions of fundamental or applied interest, and may not be only a collection of routine data, such as physical property or solubility measurements at limited pressures and temperatures close to ambient, or surfactant studies focussed strictly on micellisation or micelle structure. Papers reporting common data must be accompanied by new physical insights and/or contemporary or new theory or techniques.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


