Dynamics of heat shock protein 70 kDa in heat-shocked and hypoxic human endothelial cells
IF 3.2
3区 生物学
Q3 CELL BIOLOGY
引用次数: 0
Abstract
Heat shock proteins (HSPs) play crucial roles in human endothelial cell functions such as migration and angiogenesis. However, human HSP dynamics under stress conditions such as heat shock (HS) and hypoxia in human endothelial cells (ECs) are enigmatic, and the characteristics of HSPs in ECs after exposure to thermal stress and a low-oxygen environment are unknown. We hypothesized that ECs adapt to HS and hypoxia by modulating chaperome oligomerization and that HSP70 is a major determinant of the endothelial phenotype. HSP70 inhibition with VER-155008 or YM-1 in primary human endothelial cells decreases EC proliferation, migration, and angiogenesis at baseline and after heat shock recovery. We showed that vascular-independent HSC/P70 multimeric complexes in primary human veins (HUVEC) and coronary artery ECs (HCAEC) accumulate after HS and are decreased by hypoxia. HS recovery increases the number of HSP90 dimers, inducible HSP70, and HSP40 macromolecular complexes, whereas HSC70 returns to baseline. We demonstrated that the HS response and hypoxia regulate HSPs through a new layer of complexity, oligomerization, in addition to classical cochaperone/NEF interactions. The biphasic temporal oligomerization of molecular chaperones in the recovery phase provides a novel face of the heat shock response. In addition, shifts in the subcellular location and upregulation of HSP70 were also observed here. The decrease in HSP expression caused by hypoxia raises the possibility that decreased chaperone power contributes to the endothelial dysfunction found in atherosclerosis, thrombosis, and cancer. Together, these results show that HSP70 is pivotal to the healthy endothelial response in veins and coronary arteries, and we revealed human HSP dynamics in the vascular response to proteotoxic stress.
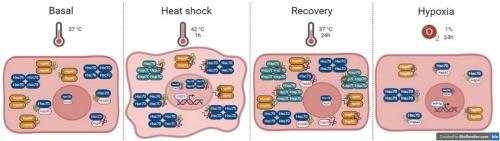
热休克蛋白70kda在热休克和缺氧人内皮细胞中的动态变化。
热休克蛋白(HSPs)在人内皮细胞迁移和血管生成等功能中起着至关重要的作用。然而,人内皮细胞(ECs)在热休克和缺氧等应激条件下的热休克蛋白动力学是谜,内皮细胞在暴露于热应激和低氧环境后的热休克蛋白特征是未知的。我们假设内皮细胞(ECs)通过调节chaperome寡聚化来适应热休克(HS)和缺氧,并且HSP70是内皮表型的主要决定因素。用VER-155008或YM-1抑制原代人内皮细胞的HSP70可降低基线和热休克恢复后EC的增殖、迁移和血管生成。我们发现,在人类初级静脉(HUVECs)和冠状动脉ECs (HCAECs)中,血管独立的HSC/P70多聚体在HS后积累,并因缺氧而减少。热休克恢复使HSP90二聚体、诱导型HSP70和HSP40大分子复合物的数量增加,而HSC70则恢复到基线水平。我们证明,除了经典的cochaperone/NEF相互作用外,热休克反应和缺氧还通过一个新的复杂性层——寡聚化来调节热休克蛋白。在恢复阶段,分子伴侣的双相时间寡聚化提供了热休克反应的新面孔。此外,亚细胞位置的变化和HSP70的上调也在这里被观察到。缺氧引起的HSP表达减少,增加了伴侣蛋白能力降低导致动脉粥样硬化、血栓形成和癌症中内皮功能障碍的可能性。总之,这些结果表明,HSP70对静脉和冠状动脉的健康内皮反应至关重要,我们揭示了人类HSP在血管对蛋白质毒性应激反应中的动态。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Stress & Chaperones
生物-细胞生物学
CiteScore
7.60
自引率
2.60%
发文量
59
审稿时长
6-12 weeks
期刊介绍:
Cell Stress and Chaperones is an integrative journal that bridges the gap between laboratory model systems and natural populations. The journal captures the eclectic spirit of the cellular stress response field in a single, concentrated source of current information. Major emphasis is placed on the effects of climate change on individual species in the natural environment and their capacity to adapt. This emphasis expands our focus on stress biology and medicine by linking climate change effects to research on cellular stress responses of animals, micro-organisms and plants.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


