The metastatic role of the CXCL10-CXCR3 axis and its therapeutic potential in osteosarcoma
IF 3.5
2区 医学
Q2 Medicine
引用次数: 0
Abstract
The CXCL10-CXCR3 axis regulates immunity, tumorigenesis, and metastasis in multiple cancers. Yet, its roles in osteosarcoma (OS), the predominant pediatric malignant bone tumor, are not fully defined. Our prior work has shown that elevated serum CXCL10 levels correlate with poor OS prognosis. The current study delves deeper by investigating how CXCL10-mediated CXCR3 signaling influences OS growth and metastatic spread. In vitro, CXCL10 and related CXCR3 ligands (CXCL4, CXCL9, and CXCL11) enhanced OS tumor cell migration. In an orthotopic xenograft mouse model with a newly created CXCR3 knockout (KO) mutant, tumor growth and lung metastasis decreased significantly when compared with the parental cell line. Transfecting the transcript isoform CXCR3A, but not CXCR3B, into KO cells restored metastatic phenotypes in mice, highlighting isoform specificity. Pharmacological CXCR3 inhibition reduced OS cell migration in vitro and metastasis in vivo. Mechanistically, CXCL10 triggered AKT (S473) and PAK1 (S144) phosphorylation in OS cell lines, but not in the KO mutant, implicating the role of these kinases in CXCL10-mediated metastasis. Collectively, our data indicate the CXCL10-CXCR3 axis as a key metastatic driver in OS, suggesting CXCR3 as a viable therapeutic target for treating OS metastasis.
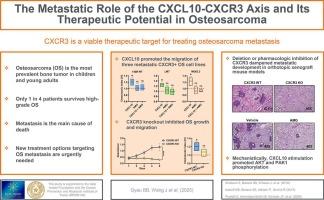
CXCL10-CXCR3轴的转移作用及其在骨肉瘤中的治疗潜力
CXCL10-CXCR3轴调节多种癌症的免疫、肿瘤发生和转移。然而,它在主要的儿童恶性骨肿瘤骨肉瘤(OS)中的作用尚未完全确定。我们之前的研究表明血清CXCL10水平升高与不良的OS预后相关。目前的研究通过研究cxcl10介导的CXCR3信号传导如何影响OS的生长和转移扩散进行了更深入的研究。在体外,CXCL10和相关的CXCR3配体(CXCL4、CXCL9和CXCL11)增强了OS肿瘤细胞的迁移。在具有新创建的CXCR3敲除(KO)突变体的原位异种移植小鼠模型中,与亲代细胞系相比,肿瘤生长和肺转移显著降低。将转录异构体CXCR3A而不是CXCR3B转染到小鼠KO细胞中,可以恢复小鼠的转移表型,突出了异构体的特异性。药理抑制CXCR3可减少OS细胞的体外迁移和体内转移。从机制上讲,CXCL10在OS细胞系中触发了AKT (S473)和PAK1 (S144)的磷酸化,但在KO突变体中没有,这暗示了这些激酶在CXCL10介导的转移中的作用。总的来说,我们的数据表明CXCL10-CXCR3轴是OS的关键转移驱动因素,表明CXCR3是治疗OS转移的可行治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Bone Oncology
ONCOLOGY-
CiteScore
7.20
自引率
2.90%
发文量
50
审稿时长
34 days
期刊介绍:
The Journal of Bone Oncology is a peer-reviewed international journal aimed at presenting basic, translational and clinical high-quality research related to bone and cancer.
As the first journal dedicated to cancer induced bone diseases, JBO welcomes original research articles, review articles, editorials and opinion pieces. Case reports will only be considered in exceptional circumstances and only when accompanied by a comprehensive review of the subject.
The areas covered by the journal include:
Bone metastases (pathophysiology, epidemiology, diagnostics, clinical features, prevention, treatment)
Preclinical models of metastasis
Bone microenvironment in cancer (stem cell, bone cell and cancer interactions)
Bone targeted therapy (pharmacology, therapeutic targets, drug development, clinical trials, side-effects, outcome research, health economics)
Cancer treatment induced bone loss (epidemiology, pathophysiology, prevention and management)
Bone imaging (clinical and animal, skeletal interventional radiology)
Bone biomarkers (clinical and translational applications)
Radiotherapy and radio-isotopes
Skeletal complications
Bone pain (mechanisms and management)
Orthopaedic cancer surgery
Primary bone tumours
Clinical guidelines
Multidisciplinary care
Keywords: bisphosphonate, bone, breast cancer, cancer, CTIBL, denosumab, metastasis, myeloma, osteoblast, osteoclast, osteooncology, osteo-oncology, prostate cancer, skeleton, tumour.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


