Novel neoadjuvant therapies for muscle-invasive bladder cancer: Systematic review and meta-analysis
Abstract
Objectives
This study aims to evaluate the efficacy and safety of novel neoadjuvant therapies including immune checkpoint inhibitors (ICI), molecular targeted agents (MTA) and antibody-drug conjugates in muscle-invasive bladder cancer through a systematic review and meta-analysis of prospective clinical trials.
Subjects/Patients and Methods
A systematic search was performed using PubMed, Web of Science, Cochrane Library, Google Scholar and ClinicalTrials.gov through December 2024. Eligible studies were phase II or higher prospective trials investigating novel agents as neoadjuvant therapy. Primary endpoints were pathologic complete response and downstaging rates. Secondary endpoints included biomarker analysis, survival outcomes and safety profiles. Data were extracted following PRISMA guidelines, and random-effects meta-analyses were performed.
Results
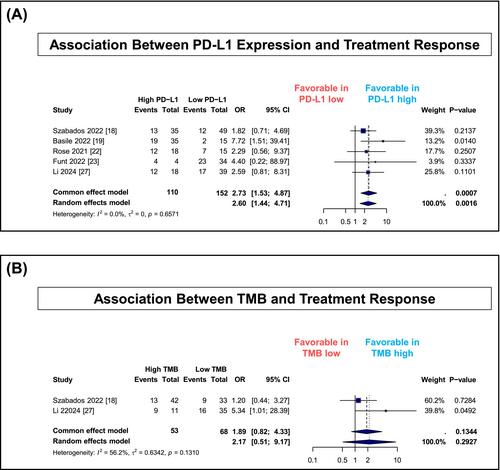
Seventeen trials comprising 1977 patients were analysed. ICI-based treatments were associated with pathologic complete response rates of 37% (95% CI, 34%–40%) and downstaging rates of 55% (95% CI, 48%–61%). ICI monotherapy was associated with higher response rates compared with MTA monotherapy (pathologic complete response: 34% vs. 5%; downstaging: 47% vs. 27%). Grade ≥3 adverse events occurred less frequently with ICI-based treatments than MTA-based treatments (35% vs. 62%). High PD-L1 expression was associated with improved pathologic response (OR, 2.60; 95% CI, 1.44–4.71). Two-year overall survival rates were 81% for ICI-based regimens and 86% for MTA-based regimens.
Conclusion
Novel neoadjuvant therapies, particularly ICI-based regimens, were associated with meaningful pathologic response rates and acceptable safety profiles. PD-L1 expression may help guide patient selection for ICI-based therapy. Randomized trials are needed to establish optimal treatment algorithms and validate predictive biomarkers.



 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


