Rebecca M. Yorker, Simon C. Deroles, Yanfei Zhou, Jennifer A. Tate, Kevin M. Davies, Nick W. Albert
{"title":"Simple and Efficient Transformation and Gene Editing of Marchantia polymorpha Spores","authors":"Rebecca M. Yorker, Simon C. Deroles, Yanfei Zhou, Jennifer A. Tate, Kevin M. Davies, Nick W. Albert","doi":"10.1002/cpz1.70149","DOIUrl":null,"url":null,"abstract":"<p><i>Marchantia polymorpha</i> (Marchantia) has become a model species for liverwort studies, owing to its rapid growth <i>in vitro</i>, ease of propagation, simple genetics, small genome, haploid-dominant life cycle, and because it is amenable to genetic transformation. Efficient transformation provides a foundation for many molecular and genetic analyses. The protocol described here is a simple and robust procedure for transforming Marchantia for a variety of applications, including gene overexpression and CRISPR genome editing. This simplified <i>Agrobacterium tumefaciens</i>-mediated transformation protocol targets spores, using common <i>Agrobacterium</i> strains GV3101 or EHA105, and overcomes challenges experienced in other methods. Spores are sterilized and distributed over sterile filter papers, which effectively retain spores and regenerating spores (known as sporelings). This approach enables the sporelings to be transferred to different agar growth media at different stages of transformation. A critical feature is preculturing the spores with acetosyringone (AS) prior to co-cultivation with <i>Agrobacterium</i>. This treatment profoundly enhances the transformation rate, particularly for <i>Agrobacterium</i> strain GV3101. GV3101 is preferred for its rapid growth rate, simple transformation, and lack of a recombinase (<i>recA</i>), stabilizing plasmids. The protocol is suitable for transforming Marchantia with constructs for CRISPR gene editing. Editing efficiency can be increased by introducing a heat-shock treatment during the transformation procedure, which increases the proportion of plants with larger edited sectors, facilitating mutant identification and propagation. Constructs and strategies for both overexpression and multiplex genome editing with sgRNA arrays using new and existing vectors are described. Using this spore transformation protocol for CRISPR gene editing, we routinely achieve 60% to 70% mutation rates, significantly reducing the effort required to generate and isolate mutants for functional analyses. © 2025 The Author(s). Current Protocols published by Wiley Periodicals LLC.</p><p><b>Basic Protocol</b>: <i>Agrobacterium</i>-mediated transformation of <i>Marchantia polymorpha</i> spores</p><p><b>Support Protocol</b>: Induction of spores</p><p><b>APPENDIX 1</b>: Overexpression Vectors</p><p><b>APPENDIX 2</b>: Multiplex CRISPR using tRNA arrays</p>","PeriodicalId":93970,"journal":{"name":"Current protocols","volume":"5 5","pages":""},"PeriodicalIF":2.2000,"publicationDate":"2025-05-27","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/cpz1.70149","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current protocols","FirstCategoryId":"1085","ListUrlMain":"https://currentprotocols.onlinelibrary.wiley.com/doi/10.1002/cpz1.70149","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 0
Abstract
Marchantia polymorpha (Marchantia) has become a model species for liverwort studies, owing to its rapid growth in vitro, ease of propagation, simple genetics, small genome, haploid-dominant life cycle, and because it is amenable to genetic transformation. Efficient transformation provides a foundation for many molecular and genetic analyses. The protocol described here is a simple and robust procedure for transforming Marchantia for a variety of applications, including gene overexpression and CRISPR genome editing. This simplified Agrobacterium tumefaciens-mediated transformation protocol targets spores, using common Agrobacterium strains GV3101 or EHA105, and overcomes challenges experienced in other methods. Spores are sterilized and distributed over sterile filter papers, which effectively retain spores and regenerating spores (known as sporelings). This approach enables the sporelings to be transferred to different agar growth media at different stages of transformation. A critical feature is preculturing the spores with acetosyringone (AS) prior to co-cultivation with Agrobacterium. This treatment profoundly enhances the transformation rate, particularly for Agrobacterium strain GV3101. GV3101 is preferred for its rapid growth rate, simple transformation, and lack of a recombinase (recA), stabilizing plasmids. The protocol is suitable for transforming Marchantia with constructs for CRISPR gene editing. Editing efficiency can be increased by introducing a heat-shock treatment during the transformation procedure, which increases the proportion of plants with larger edited sectors, facilitating mutant identification and propagation. Constructs and strategies for both overexpression and multiplex genome editing with sgRNA arrays using new and existing vectors are described. Using this spore transformation protocol for CRISPR gene editing, we routinely achieve 60% to 70% mutation rates, significantly reducing the effort required to generate and isolate mutants for functional analyses. © 2025 The Author(s). Current Protocols published by Wiley Periodicals LLC.
Basic Protocol: Agrobacterium-mediated transformation of Marchantia polymorpha spores
Support Protocol: Induction of spores
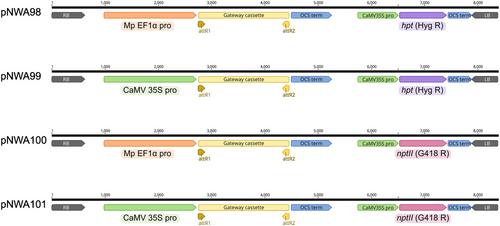
APPENDIX 1: Overexpression Vectors
APPENDIX 2: Multiplex CRISPR using tRNA arrays








 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


