Patient phenotyping for molecular profiling of neck and low back pain – Study protocol
Q2 Medicine
引用次数: 0
Abstract
Background
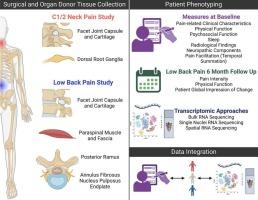
Chronic neck and low back pain are highly prevalent, leading causes of disability, and associated with long-term opioid use. The development of effective therapeutics is hampered by the limited understanding of the molecular mechanisms underlying these conditions. The Human Nociceptor and Spinal Cord Molecular Signature Center is a consortium within the NIH PRECISION Human Pain Network. The Center aims to fundamentally advance the understanding of the molecular neurobiology and neuroimmunology underlying human neck and low back pain, thereby enabling the discovery of therapeutic targets. We are pursuing this aim by applying bulk, single cell and spatial transcriptomics to tissues recovered from patients with neck and low back pain undergoing C1-2 and lumbar arthrodesis. The C2 dorsal root ganglion, facet joints, muscles, fascia, and intervertebral discs are harvested; control tissues are obtained from organ donors. A critical advantage of human research is the study of molecular neurobiological mechanisms in the context of the phenotypic complexity of chronic pain. The aim of this article is to summarize the rationale and methods used in our project to phenotype patients.
Methods
Phenotyping domains include pain-related characteristics such as pain intensity, duration, and location; physical function; psychosocial function; neuropathic components assessed by self-report and quantitative sensory testing; somatosensory functions such as mechanical pain sensitivity and temporal summation; and radiological findings.
Conclusion
We anticipate that comprehensive phenotyping will greatly facilitate the identification of phenotype-specific transcriptional signatures associated with chronic neck and low back pain, revealing new neurobiological and/or neuro-immunological mechanisms of painful diseases.
颈部和腰痛分子谱的患者表型分析-研究方案
慢性颈部和下背部疼痛非常普遍,是致残的主要原因,并与长期使用阿片类药物有关。由于对这些疾病的分子机制了解有限,有效治疗方法的发展受到阻碍。人类伤害感受器和脊髓分子特征中心是美国国立卫生研究院精密人类疼痛网络的一个联盟。该中心旨在从根本上推进对人类颈部和腰痛的分子神经生物学和神经免疫学的理解,从而发现治疗靶点。我们正在通过将大体积、单细胞和空间转录组学应用于接受C1-2和腰椎关节融合术的颈部和腰痛患者恢复的组织来实现这一目标。切除C2背根神经节、小关节、肌肉、筋膜和椎间盘;对照组织来自器官捐献者。人类研究的一个关键优势是在慢性疼痛表型复杂性的背景下研究分子神经生物学机制。本文的目的是总结在我们的项目的基本原理和方法,以表型患者。方法分型域包括疼痛相关特征,如疼痛强度、持续时间和部位;生理功能;社会心理功能;用自我报告和定量感觉测试评估神经病变成分;躯体感觉功能,如机械疼痛敏感性和时间累加;还有放射检查结果。结论全面的表型分析将极大地促进慢性颈、腰痛相关表型特异性转录特征的识别,揭示疼痛性疾病的新的神经生物学和/或神经免疫学机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neurobiology of Pain
Medicine-Anesthesiology and Pain Medicine
CiteScore
4.40
自引率
0.00%
发文量
29
审稿时长
54 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


