Transcriptomic characterization of the synergy between human induced pluripotent stem cells-derived liver- and pancreas-on-chip coculture
IF 3.6
3区 医学
Q2 CELL BIOLOGY
引用次数: 0
Abstract
Interactions between the liver and pancreas are key features of the carbohydrate and lipid homeostasis in healthy and pathological patients. To investigate the crosstalk between the two organs, we have developed an organ-on-chip coculture model derived from human induced pluripotent stem cells. The presence of pancreatic-derived tissue in the culture environment contributed to increase the CYP3A4 activity, the glycogen storage, and the expression of genes related to lipids, bile acids and sterol metabolism in the liver derived tissue. Concomitantly, the presence of liver cells led to increase the C-peptide secretion in pancreas. The coculture with liver modulated the pancreatic differentiation by increasing the activity of important transcription factors (REST, MAFB, PBX1) and by downregulating several hormone encoding genes (INS, GCG, TTR). The liver also stimulated the expression of genes involved in the response to inflammation in pancreas (via TGFβ/SMAD pathway). In parallel we observed a pancreatic cell reorganization coupled with the activation of the cell proliferation related transcription factor (SCRT1) and the upregulation of cellular remodeling genes (FLNA, FLNB, FN1, COL4A5). Finally, the pancreatic lipid genes were also upregulated in presence of the liver tissue. Overall, our results reflect a complex synergy between both tissues. We believe that those results are an encouraging step toward the development of relevant human model using advanced organ-on-chip technology and stem cells sources.
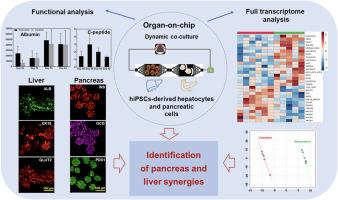
人诱导多能干细胞衍生的肝脏和胰腺芯片共培养之间协同作用的转录组学表征
肝脏和胰腺之间的相互作用是健康和病理患者碳水化合物和脂质稳态的关键特征。为了研究这两个器官之间的相互作用,我们开发了一种源自人类诱导多能干细胞的器官芯片共培养模型。胰源性组织在培养环境中的存在增加了肝源性组织中CYP3A4活性、糖原储存以及与脂质、胆汁酸和固醇代谢相关基因的表达。同时,肝细胞的存在导致胰腺c肽分泌增加。与肝脏共培养通过增加重要转录因子(REST、MAFB、PBX1)的活性和下调几个激素编码基因(INS、GCG、TTR)来调节胰腺分化。肝脏还刺激了参与胰腺炎症反应的基因的表达(通过TGFβ/SMAD途径)。同时,我们观察到胰腺细胞重组伴随着细胞增殖相关转录因子(SCRT1)的激活和细胞重塑基因(FLNA, FLNB, FN1, COL4A5)的上调。最后,胰腺脂质基因在肝组织的存在下也上调。总的来说,我们的结果反映了两个组织之间复杂的协同作用。我们相信,这些结果是朝着利用先进的器官芯片技术和干细胞来源开发相关人体模型迈出的令人鼓舞的一步。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular and Cellular Endocrinology
医学-内分泌学与代谢
CiteScore
9.00
自引率
2.40%
发文量
174
审稿时长
42 days
期刊介绍:
Molecular and Cellular Endocrinology was established in 1974 to meet the demand for integrated publication on all aspects related to the genetic and biochemical effects, synthesis and secretions of extracellular signals (hormones, neurotransmitters, etc.) and to the understanding of cellular regulatory mechanisms involved in hormonal control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


