Multicenter phase Ib/II study of second-line durvalumab and tremelimumab in combination with paclitaxel in patients with biomarker-selected metastatic gastric cancer
IF 6.8
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
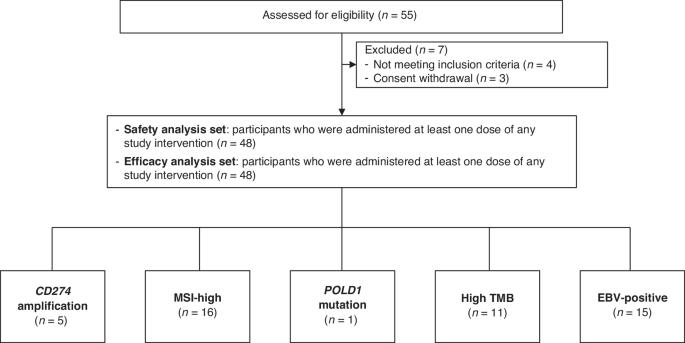
This multicenter phase Ib/II trial aimed to evaluate the safety and efficacy of combining durvalumab, tremelimumab, and paclitaxel as second-line treatment for biomarker-selected patients with metastatic gastric cancer. In phase Ib, the standard 3 + 3 dose escalation method was used. Durvalumab and tremelimumab were administered every 4 weeks for 13 and 4 cycles, respectively, combining paclitaxel 80 mg/m2 (dose level 2) or 60 mg/m2 (dose level 1) on days 1, 8, and 15. The primary outcome for phase II was the objective response rate (ORR). In phase Ib (n = 7), dose level-1 was selected as the recommended phase II dose. In phase II, 48 patients were enrolled: microsatellite instability-high or deficient mismatch repair protein tumors (n = 16); EBV-positive tumors (n = 15); high tumor mutation burden ( ≥ 5/Mb) (n = 11); CD274 amplification (n = 5); and POLD1 mutation (n = 1). The ORR was 52.1%, meeting the primary endpoint. The median progression-free survival and overall survival were 5.3 and 13.1 months, respectively. The most common any-grade and grade 3–4 adverse events were anemia (41.7%) and neutropenia (10.4%), respectively. Durvalumab-tremelimumab with paclitaxel was tolerable and efficacious in biomarker-selected gastric cancer patients as a second-line treatment, highlighting the importance of biomarker-based approaches for immunotherapy in gastric cancer. NCT03751761.
二线durvalumab和tremelimumab联合紫杉醇治疗生物标志物选择性转移性胃癌的多中心Ib/II期研究
背景:这项多中心Ib/II期临床试验旨在评估联合durvalumab、tremelimumab和紫杉醇作为生物标志物选择的转移性胃癌患者的二线治疗的安全性和有效性。方法:Ib期采用标准的3 + 3剂量递增法。Durvalumab和tremelimumab每4周给药一次,分别为13和4个周期,在第1、8和15天联合紫杉醇80mg /m2(剂量水平2)或60mg /m2(剂量水平1)。II期的主要终点是客观缓解率(ORR)。结果:在Ib期(n = 7)中,剂量水平1被选择为推荐的II期剂量。在II期,48例患者入组:微卫星不稳定性高或缺陷错配修复蛋白肿瘤(n = 16);ebv阳性肿瘤(n = 15);高肿瘤突变负荷(≥5/Mb) (n = 11);CD274扩增(n = 5);POLD1突变(n = 1)。ORR为52.1%,达到主要终点。中位无进展生存期和总生存期分别为5.3个月和13.1个月。最常见的任何级别和3-4级不良事件分别是贫血(41.7%)和中性粒细胞减少(10.4%)。结论:Durvalumab-tremelimumab联合紫杉醇在生物标志物选择的胃癌患者中作为二线治疗是耐受和有效的,突出了基于生物标志物的方法在胃癌免疫治疗中的重要性。临床试验注册:NCT03751761。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

British Journal of Cancer
医学-肿瘤学
CiteScore
15.10
自引率
1.10%
发文量
383
审稿时长
6 months
期刊介绍:
The British Journal of Cancer is one of the most-cited general cancer journals, publishing significant advances in translational and clinical cancer research.It also publishes high-quality reviews and thought-provoking comment on all aspects of cancer prevention,diagnosis and treatment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


