Cannabinol (CBN) alleviates age-related cognitive decline by improving synaptic and mitochondrial health
IF 11.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
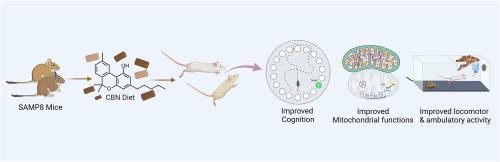
Age-related cognitive decline and neurodegenerative diseases, such as Alzheimer's disease, represent major global health challenges, particularly with an aging population. Mitochondrial dysfunction appears to play a central role in the pathophysiology of these conditions by driving redox dysregulation and impairing cellular energy metabolism. Despite extensive research, effective therapeutic options remain limited. Cannabinol (CBN), a cannabinoid previously identified as a potent inhibitor of oxytosis/ferroptosis through mitochondrial modulation, has demonstrated promising neuroprotective effects. In cell culture, CBN targets mitochondria, preserving mitochondrial membrane potential, enhancing antioxidant defenses and regulating bioenergetic processes. However, the in vivo therapeutic potential of CBN, particularly in aging models, has not been thoroughly explored. To address this gap, this study investigated the effects of CBN on age-associated cognitive decline and metabolic dysfunction using the SAMP8 mouse model of accelerated aging. Our results show that CBN significantly improves spatial learning and memory, with more pronounced cognitive benefits observed in female mice. These cognitive improvements are accompanied by sex-specific changes in metabolic parameters, such as enhanced oxygen consumption and energy expenditure. Mechanistically, CBN modulates key regulators of mitochondrial dynamics, including mitofusin 2 (MFN2) and dynamin-related protein 1 (DRP1), while upregulating markers of mitochondrial biogenesis including mitochondrial transcription factor A (TFAM) and translocase of outer mitochondrial membrane 20 (TOM20). Additionally, CBN upregulates key synaptic proteins involved in vesicle trafficking and postsynaptic signaling suggesting that it enhances synaptic function and neurotransmission, further reinforcing its neuroprotective effects. This study provides in vivo evidence supporting CBN's potential to mitigate age-related cognitive and metabolic dysfunction, with notable sex-specific effects, highlighting its promise for neurodegenerative diseases and cognitive decline.
大麻酚(CBN)通过改善突触和线粒体健康来缓解与年龄相关的认知衰退
与年龄有关的认知能力下降和神经退行性疾病,如阿尔茨海默病,是主要的全球健康挑战,特别是在人口老龄化的情况下。线粒体功能障碍似乎通过驱动氧化还原失调和损害细胞能量代谢在这些疾病的病理生理学中发挥核心作用。尽管进行了广泛的研究,但有效的治疗选择仍然有限。大麻酚(CBN)是一种大麻素,以前被认为是一种有效的通过线粒体调节的氧中毒/铁中毒抑制剂,已经证明了有希望的神经保护作用。在细胞培养中,CBN靶向线粒体,保护线粒体膜电位,增强抗氧化防御和调节生物能量过程。然而,CBN的体内治疗潜力,特别是在衰老模型中,尚未被彻底探索。为了解决这一空白,本研究利用加速衰老的SAMP8小鼠模型研究了CBN对年龄相关认知能力下降和代谢功能障碍的影响。我们的研究结果表明,CBN可以显著改善空间学习和记忆,在雌性小鼠中观察到更明显的认知益处。这些认知能力的改善伴随着代谢参数的性别特异性变化,如氧气消耗和能量消耗的增加。在机制上,CBN调节线粒体动力学的关键调节因子,包括mitofusin 2 (MFN2)和动力蛋白相关蛋白1 (DRP1),同时上调线粒体生物发生标志物,包括线粒体转录因子A (TFAM)和线粒体外膜转位酶20 (TOM20)。此外,CBN上调参与囊泡运输和突触后信号传导的关键突触蛋白,表明其增强突触功能和神经传递,进一步增强其神经保护作用。该研究提供了体内证据,支持CBN具有减轻年龄相关认知和代谢功能障碍的潜力,并具有显著的性别特异性效应,突出了其对神经退行性疾病和认知能力下降的前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


