[Simultaneous determination of 10 phthalate metabolites in urine by ultra performance liquid chromatography -tandem mass spectrometry].
Abstract
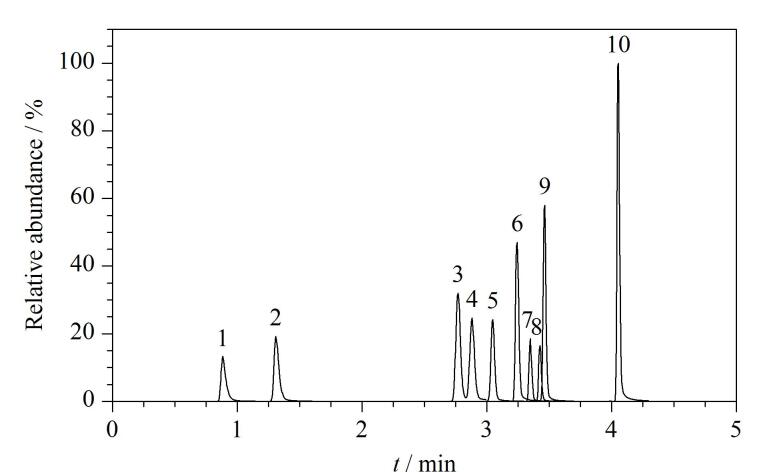
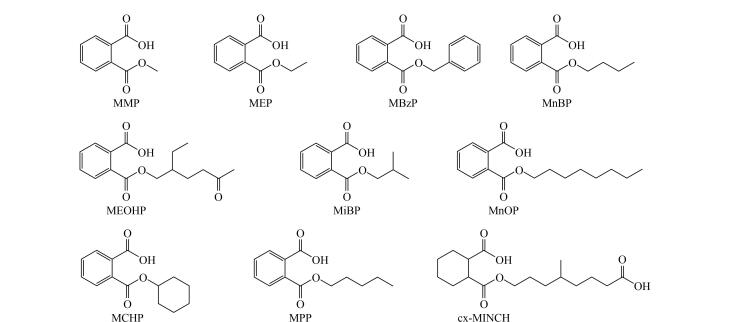
Phthalates (PAEs) are widely employed as plasticizers in plastic products that are used in industrial, agricultural, food, medical, and other fields. PAEs are relatively weakly bonded to plastic products through non-covalent interactions. Consequently, PAEs can easily leak from the product into the environment, which exposes the public to PAEs through food intake, skin absorption from personal care products, and by inhaling air. Related studies have shown that PAEs are endocrine-disrupting substances and that long-term exposure to PAEs may result in diseases of the nervous, reproductive, cardiovascular and immune systems. In addition, excessive exposure to PAEs may trigger inflammatory responses and induce tumors. Therefore, establishing a highly sensitive assay for determining PAE levels in the human body following exposure is an important objective. PAEs generally have half-lives of less than 24 h; they are rapidly metabolized through enzymatic hydrolysis after entering the human body and excreted through urine. Therefore, most studies have focused on PAE metabolites as target compounds; hence, human body exposure to PAEs can be assessed by analyzing the types and levels of these metabolites. Herein, we established a method for simultaneously determining ten phthalate (PAE) metabolites in human urine using ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). The ten PAE metabolites in urine were separated using an ACQUITY UPLC BEH Phenyl column (50 mm×2.1 mm, 1.7 μm). Gradient elution was performed using 0.1% formic acid aqueous solution and 0.1% formic acid in acetonitrile as the mobile phases, at a flow rate of 0.5 mL/min, a column temperature of 40 ℃, and a sample size of 20 μL. Data were acquired in negative-ion electrospray ionization (ESI) and multiple reaction monitoring (MRM) modes, and quantified using the isotope internal standard method. The method was found to be highly specific, with the ten PAE metabolites exhibiting good linearities in their linear ranges, with limits of detection (LODs) and quantification (LOQs) of 0.03-0.3 and 0.1-1 ng/mL, respectively. Under the four quality control (QC) levels, the intra-day and inter-day precisions of the ten PAE metabolites were all ≤8.3%, and the accuracy ranged from ‒10.5% to 7.3%. The method was used to assess the exposure levels of PAE metabolites in the urine samples of 60 volunteers, with 1‒6 kinds of PAE metabolites detected in the urine of each volunteer. This method is sensitive, accurate, simple, efficient, and suitable for the large-scale biological monitoring of PAE metabolites.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


