Hemorrhagic and ischemic risks of anti-VEGF therapies in glioblastoma
IF 5
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
Glioblastoma (GBM) is one of the most aggressive primary brain tumors, characterized by extensive neovascularization and a highly infiltrative phenotype. Anti-vascular endothelial growth factor (VEGF) therapies, such as bevacizumab, have emerged as significant adjunct treatments for recurrent and high-grade GBM by targeting abnormal tumor vasculature. Despite demonstrated benefits in slowing tumor progression and alleviating peritumoral edema, these agents are associated with notable vascular complications, including hemorrhagic and ischemic events. Hemorrhagic complications range from minor intracranial microbleeds to life-threatening intracranial hemorrhages (ICH). Mechanistically, VEGF inhibition disrupts endothelial function and decreases vascular integrity, making already fragile tumor vessels prone to rupture. Glioma-associated vascular abnormalities, including disorganized vessel networks and compromised blood-brain barrier, further exacerbate bleeding risks. Concurrent use of anticoagulants, hypertension, and genetic predispositions also significantly elevate hemorrhagic risk. In addition to bleeding complications, ischemic events are increasingly recognized in patients receiving anti-VEGF therapy. Reduced vascular endothelial cells (ECs) survival and diminished microvascular density can lead to regional hypoperfusion and potentially precipitate cerebrovascular ischemia. Impaired vasoreactivity and increased vascular resistance, often accompanied by endothelial dysfunction and microvascular rarefaction, contribute to elevated stroke and arterial thrombotic risk. This review synthesizes current evidence on hemorrhagic and ischemic complications arising from anti-VEGF therapy in GBM. We discuss underlying pathophysiological mechanisms, risk factors, and clinically relevant biomarkers, as well as prevention strategies—such as rigorous blood pressure (BP) control and close monitoring of coagulation parameters. We further highlight emerging avenues in precision medicine, including pharmacogenomic profiling and targeted adjunct agents that protect vascular integrity, aimed at mitigating adverse vascular events while preserving therapeutic efficacy. The goal is to optimize outcomes for GBM patients by balancing the benefits of anti-VEGF therapy with vigilant management of its inherent vascular risks. In addition, this study analyzes existing clinical trials of the use of anti-VEGF drugs in the treatment of gliomas using data from the clinicaltirals.gov website.
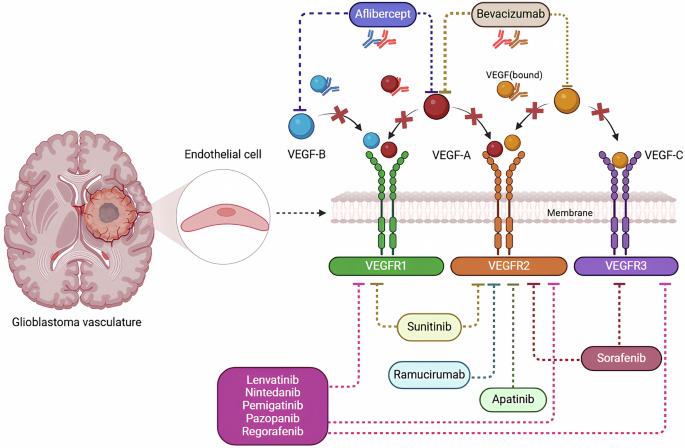
胶质母细胞瘤中抗vegf治疗的出血和缺血风险。
胶质母细胞瘤(GBM)是最具侵袭性的原发性脑肿瘤之一,其特点是广泛的新生血管和高度浸润的表型。抗血管内皮生长因子(VEGF)疗法,如贝伐单抗,通过靶向异常肿瘤血管系统,已成为复发性和高级别GBM的重要辅助治疗。尽管这些药物在减缓肿瘤进展和减轻肿瘤周围水肿方面具有益处,但它们与明显的血管并发症相关,包括出血和缺血事件。出血并发症的范围从轻微的颅内微出血到危及生命的颅内出血(ICH)。从机制上讲,VEGF抑制破坏内皮功能,降低血管完整性,使本已脆弱的肿瘤血管容易破裂。胶质瘤相关的血管异常,包括血管网络紊乱和血脑屏障受损,进一步加剧出血风险。同时使用抗凝剂、高血压和遗传易感性也会显著增加出血风险。除了出血并发症外,在接受抗vegf治疗的患者中,缺血性事件也越来越被认识到。血管内皮细胞(ECs)存活率降低和微血管密度降低可导致局部灌注不足,并可能诱发脑血管缺血。血管反应性受损和血管阻力增加,常伴有内皮功能障碍和微血管稀疏,导致卒中和动脉血栓形成风险升高。本文综述了目前关于抗vegf治疗GBM引起的出血和缺血性并发症的证据。我们讨论了潜在的病理生理机制、危险因素和临床相关的生物标志物,以及预防策略,如严格控制血压和密切监测凝血参数。我们进一步强调了精准医学的新兴途径,包括药物基因组分析和保护血管完整性的靶向辅助剂,旨在减轻血管不良事件,同时保持治疗效果。目标是通过平衡抗vegf治疗的益处和对其固有血管风险的警惕管理来优化GBM患者的预后。此外,本研究利用clinicaltirals.gov网站的数据,分析了使用抗vegf药物治疗胶质瘤的现有临床试验。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


