Differential effect of targeting cisplatin-induced nitrative stress using MnTBAP in auditory and cancer cells
IF 2.9
Q2 TOXICOLOGY
引用次数: 0
Abstract
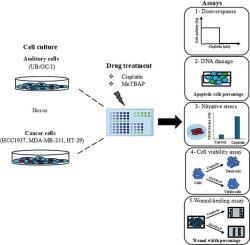
Ototoxicity is a major dose-limiting side effect of cisplatin, a highly effective anti-cancer drug used to treat many solid tumors. Oxidative stress plays a central role in mediating cisplatin-induced ototoxicity. However, broad-spectrum antioxidants that prevent ototoxicity compromise the anti-cancer activity of cisplatin. Therefore, there is a need to identify novel interventional targets/compounds for otoprotection. Recent reports indicated that cisplatin-induced nitration of cochlear proteins is a critical factor in causing ototoxicity, and inhibition of cochlear nitrative stress mitigated cisplatin-induced ototoxicity. The use of peroxynitrite decomposition catalysts that selectively target nitrative stress appears to be an attractive strategy for mitigating the ototoxic effects of cisplatin because they do not scavenge free radicals. We hypothesized that cotreatment with selective inhibitors of nitrative stress prevents cisplatin-induced ototoxicity without compromising the anti-cancer effects. Here, we test this hypothesis by investigating the effect of MnTBAP cotreatment on cell viability, nitrative stress, DNA damage, and cell migration in cisplatin-treated organ of Corti as well as cancer cells. Our results indicate that cisplatin treatment decreases cell viability in both auditory and cancer cells, while cotreatment with MnTBAP mitigates cisplatin-induced cytotoxicity in the auditory cells but not in the cancer cells. Collectively, the findings of this study suggest that selective targeting of cisplatin-induced nitrative stress is a promising strategy for mitigating the ototoxic effects of cisplatin because it does not compromise the anti-cancer effects.
利用mnttap靶向顺铂诱导的听觉细胞和癌细胞的硝化应激的差异效应
耳毒性是顺铂的主要剂量限制副作用,顺铂是一种非常有效的抗癌药物,用于治疗许多实体瘤。氧化应激在介导顺铂诱导的耳毒性中起核心作用。然而,防止耳毒性的广谱抗氧化剂损害了顺铂的抗癌活性。因此,有必要确定新的耳保护干预靶点/化合物。最近的报道表明,顺铂诱导的耳蜗蛋白硝化是引起耳毒性的关键因素,抑制耳蜗硝化应激可减轻顺铂诱导的耳毒性。使用选择性靶向硝化应激的过氧亚硝酸盐分解催化剂似乎是减轻顺铂耳毒性作用的一种有吸引力的策略,因为它们不清除自由基。我们假设与选择性硝酸应激抑制剂共同治疗可以防止顺铂诱导的耳毒性,而不会影响抗癌效果。在这里,我们通过研究MnTBAP共处理对顺铂处理的Corti器官以及癌细胞的细胞活力、营养应激、DNA损伤和细胞迁移的影响来验证这一假设。我们的研究结果表明,顺铂治疗降低了听觉细胞和癌细胞的细胞活力,而与MnTBAP共治疗减轻了顺铂诱导的听觉细胞的细胞毒性,而不是癌细胞。总的来说,本研究的结果表明,选择性靶向顺铂诱导的硝化应激是减轻顺铂耳毒性作用的一种有希望的策略,因为它不会损害抗癌作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Current Research in Toxicology
Environmental Science-Health, Toxicology and Mutagenesis
CiteScore
4.70
自引率
3.00%
发文量
33
审稿时长
82 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


