Nuclear SREBP2 condensates regulate the transcriptional activation of lipogenic genes and cholesterol homeostasis
IF 20.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
The precursor of sterol regulatory element-binding protein-2 (SREBP2) is a membrane-bound transcription factor regulating cholesterol biosynthesis. Under cholesterol-deficient conditions, mature SREBP2 is released from membrane-bound precursors through proteolytic cleavage and enters the nucleus. However, regulation of the transcriptional activity of nuclear SREBP2 (nSREBP2) is poorly understood. In the present study, we reported that nSREBP2 forms nuclear condensates through its amino-terminal, intrinsically disordered region (IDR) and works together with transcription coactivators, partly on superenhancers, for the transcriptional activation of SREBP2 target genes. Substitution of a conserved phenylalanine by alanine within the IDR abolishes the formation of nSREBP2 condensates and reduces its transcriptional activity. This can be effectively rescued by fusion with a phase separation driving FUS-IDR. Knock-in of the phenylalanine-to-alanine substitution in male mice compromises feeding-induced nSREBP2 activity and lowers hepatic and circulating cholesterol levels, underscoring the functional significance of nSREBP2 condensates. Together, the present study reveals that nuclear condensates driven by nSREBP2 N-terminal IDR facilitate the efficient activation of lipogenic genes and play an important role in cholesterol homeostasis. Nuclear SREBP2 forms nuclear condensates through its N-terminal intrinsically disordered region, which is essential for the transcriptional activation of lipogenic genes.
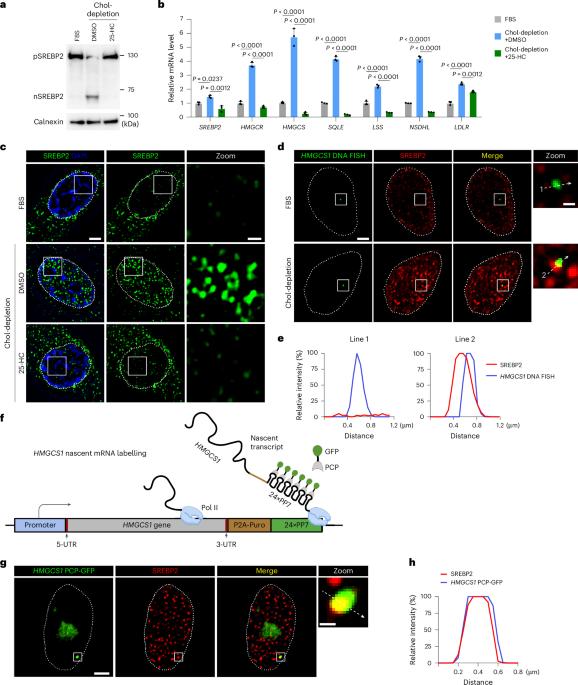

核SREBP2凝聚体调节脂肪生成基因的转录激活和胆固醇稳态
甾醇调节元件结合蛋白-2 (SREBP2)的前体是一种调节胆固醇生物合成的膜结合转录因子。在胆固醇缺乏的情况下,成熟的SREBP2通过蛋白水解裂解从膜结合的前体中释放出来并进入细胞核。然而,细胞核SREBP2 (nSREBP2)转录活性的调控机制尚不清楚。在本研究中,我们报道了nSREBP2通过其氨基末端,内在无序区(IDR)形成核凝聚物,并与转录共激活因子(部分在超增强子上)共同作用,以激活SREBP2靶基因的转录。IDR内的丙氨酸取代保守的苯丙氨酸可消除nSREBP2凝聚物的形成并降低其转录活性。这可以通过与驱动FUS-IDR的相分离融合有效地挽救。在雄性小鼠中,苯丙氨酸到丙氨酸替代的敲入降低了喂养诱导的nSREBP2活性,降低了肝脏和循环胆固醇水平,强调了nSREBP2凝聚物的功能意义。综上所述,本研究揭示了nSREBP2 n端IDR驱动的核凝析物促进了脂肪生成基因的有效激活,并在胆固醇稳态中发挥了重要作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


