TNFα Receptor 1 and Not Receptor 2 Affect Annulus Fibrosus and Nucleus Pulposus Response to Cytokine Challenge in a Rat Model
Abstract
Background
Painful intervertebral disc (IVD) degeneration (IVDD) involves chronic inflammation. Developing translational immunomodulatory strategies for IVDD is a priority with tumor necrosis factor alpha (TNFα) signaling an important target. TNFα binds to 2 receptors (TNFRs), with TNFR1 signaling promoting catabolism and apoptosis and TNFR2 signaling promoting anabolism and proliferation.
Methods
This study developed translational strategies to evaluate and modulate TNFR1 and TNFR2 signaling in rat in vivo and in vitro IVDD models. We used blocking antibodies, the TNFR2-activator Atsttrin, and small molecule inhibitors of TNFR1 to discern distinct TNFR1 and TNFR2-effects on annulus fibrosus (AF) and nucleus pulposus (NP) cells and to identify effective strategies for modulating specific TNFRs.
Results
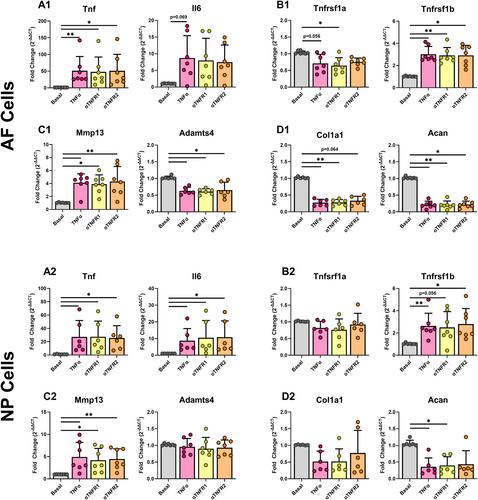
TNFR1 was significantly increased with IVDD in vivo in the NP while TNFR2 was unaffected with very faint staining. TNFR1-specific small molecule inhibitors were effective in reducing catabolic effects of TNFα, highlighting the efficacy of this small molecule strategy for TNFR1 signaling modulation. Meanwhile, TNFR1 and TNFR2 inhibition in vitro was not effective with blocking antibodies on NP or AF cells, likely due to species-specificity of available blocking antibodies. Further, TNFR2 activation with Atsttrin was similarly ineffective, likely due to extremely low TNFR2 levels in both AF and NP cells.
Conclusions
TNFα receptor-specific signaling is important in rat IVDD in vivo and in vitro. TNFR1 inhibition was more effective with small molecules than using blocking antibodies. Low levels of TNFR2 in rat AF and NP cells and lack of efficacy of TNFR2-activator Atsttrin suggest native AF and NP cells have little capacity for TNFR2-dependent IVD repair.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


