Implementing CYP2C19-guided clopidogrel therapy: a scoping review of pharmacogenomic testing services
IF 2.9
3区 医学
Q2 GENETICS & HEREDITY
引用次数: 0
Abstract
Pharmacogenomic testing for CYP2C19 helps personalise clopidogrel therapy and reduces the risk of experiencing a secondary myocardial infarction in individuals with impaired CYP2C19 function. Routine testing, however, is uncommon and it is proposed that the key requirements and processes of testing services are poorly understood. This scoping review aimed to explore the literature for CYP2C19 testing services for clopidogrel and identify their commonalities to inform the design and delivery of future services. In total, 37 eligible studies describing services across hospital and community settings were retrieved. Key elements of delivery included a multi-disciplinary approach involving physicians and pharmacists, provision of pre-implementation training and education, and electronic communication of test results. Result integration into clinical decision support systems improved the practical application of pharmacogenomic testing. The identification of the key requirements and processes may be used by institutions looking to design and deliver CYP2C19 testing services to guide clopidogrel therapy.
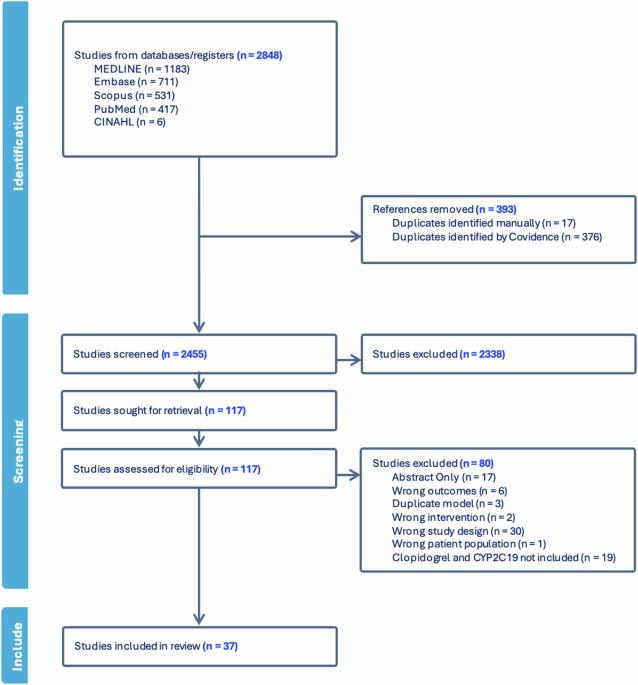
实施cyp2c19引导的氯吡格雷治疗:药物基因组学检测服务的范围综述
CYP2C19药物基因组学检测有助于氯吡格雷个体化治疗,降低CYP2C19功能受损患者继发性心肌梗死的风险。然而,常规测试是不常见的,并且提出测试服务的关键要求和过程理解得很差。本综述旨在探讨氯吡格雷CYP2C19检测服务的文献,并确定其共性,为未来服务的设计和提供提供参考。总共检索了37项描述医院和社区环境服务的合格研究。交付的关键要素包括医生和药剂师参与的多学科方法,提供实施前培训和教育,以及测试结果的电子通信。结果与临床决策支持系统的整合提高了药物基因组学检测的实际应用。关键要求和流程的确定可用于寻求设计和提供CYP2C19检测服务的机构,以指导氯吡格雷治疗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Pharmacogenomics Journal
医学-药学
CiteScore
7.20
自引率
0.00%
发文量
35
审稿时长
6-12 weeks
期刊介绍:
The Pharmacogenomics Journal is a print and electronic journal, which is dedicated to the rapid publication of original research on pharmacogenomics and its clinical applications.
Key areas of coverage include:
Personalized medicine
Effects of genetic variability on drug toxicity and efficacy
Identification and functional characterization of polymorphisms relevant to drug action
Pharmacodynamic and pharmacokinetic variations and drug efficacy
Integration of new developments in the genome project and proteomics into clinical medicine, pharmacology, and therapeutics
Clinical applications of genomic science
Identification of novel genomic targets for drug development
Potential benefits of pharmacogenomics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


