Effect of inosine on recurrence of tumor after radiation therapy: A mathematical investigation
IF 2
4区 数学
Q2 BIOLOGY
引用次数: 0
Abstract
Tumor invasion that marks the transition from localized growth to aggressive spread is critical in cancer biology. It involves the breakdown of surrounding tissues through matrix degradation enzymes, such as urokinase plasminogen activator and matrix metalloproteinases, to promote cancer cells migration. Understanding invasion pathways is crucial for developing effective therapies and improving patient outcomes. There is a significant progress in cancer treatments; however, the treatments such as radiotherapy and chemotherapy are ineffective many times in the sense that some cancer cells survive, leading to tumor recurrence. It has been observed that dead cells play a key role in cancer recurrence. The dead cells, particularly due to treatments like radiation or chemotherapy, release signals and cellular components, including nucleotides like inosine, cytokines, and growth factors. These factors influence the tumor microenvironment and promote the survival and proliferation of nearby cancer cells. In this article, a novel mathematical model is proposed to investigate the effects of inosine on tumor recurrence and invasion. The simulated results are in very good agreement with the experimental and numerical results of the literature. The model simulated the inosine generation after some time of radiotherapy treatment withdrawal, and it achieves a log-normal profile. In line of experimental observations, it is obtained that the cancer cells proliferate with usual proliferation but once inosine gets generated from the dead cells, the proliferation intensity of cancer cells is enhanced significantly.
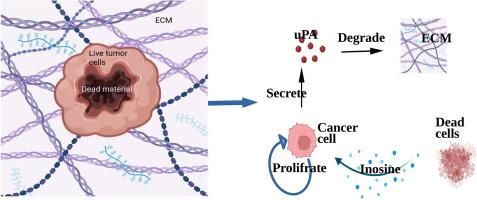
肌苷对放射治疗后肿瘤复发的影响:一个数学研究。
肿瘤侵袭标志着从局部生长到侵袭性扩散的转变,在癌症生物学中是至关重要的。它涉及通过基质降解酶,如尿激酶纤溶酶原激活剂和基质金属蛋白酶,破坏周围组织,促进癌细胞迁移。了解入侵途径对于开发有效的治疗方法和改善患者预后至关重要。癌症治疗取得了重大进展;然而,放疗和化疗等治疗方法在很多时候是无效的,因为一些癌细胞存活了下来,导致肿瘤复发。据观察,死细胞在癌症复发中起关键作用。死细胞,特别是由于放疗或化疗等治疗,释放信号和细胞成分,包括核苷酸,如肌苷,细胞因子和生长因子。这些因素影响肿瘤微环境,促进附近癌细胞的生存和增殖。本文提出了一个新的数学模型来研究肌苷对肿瘤复发和侵袭的影响。模拟结果与文献的实验和数值结果吻合得很好。该模型模拟了放疗停药一段时间后肌苷的生成,达到对数正态分布。通过实验观察发现,癌细胞的增殖是正常的,但一旦死细胞产生肌苷,癌细胞的增殖强度就会明显增强。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
4.20
自引率
5.00%
发文量
218
审稿时长
51 days
期刊介绍:
The Journal of Theoretical Biology is the leading forum for theoretical perspectives that give insight into biological processes. It covers a very wide range of topics and is of interest to biologists in many areas of research, including:
• Brain and Neuroscience
• Cancer Growth and Treatment
• Cell Biology
• Developmental Biology
• Ecology
• Evolution
• Immunology,
• Infectious and non-infectious Diseases,
• Mathematical, Computational, Biophysical and Statistical Modeling
• Microbiology, Molecular Biology, and Biochemistry
• Networks and Complex Systems
• Physiology
• Pharmacodynamics
• Animal Behavior and Game Theory
Acceptable papers are those that bear significant importance on the biology per se being presented, and not on the mathematical analysis. Papers that include some data or experimental material bearing on theory will be considered, including those that contain comparative study, statistical data analysis, mathematical proof, computer simulations, experiments, field observations, or even philosophical arguments, which are all methods to support or reject theoretical ideas. However, there should be a concerted effort to make papers intelligible to biologists in the chosen field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


