Ex vivo machine perfusion as a platform for lentiviral gene delivery in rat livers
IF 4.5
3区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Developing new strategies for local monitoring and delivery of immunosuppression is critical to making allografts safer and more accessible. Ex vivo genetic modification of grafts using machine perfusion presents a promising approach to improve graft function and modulate immune responses while minimizing risks of off-target effects and systemic immunogenicity in vivo. This proof-of-concept study demonstrates the feasibility of using normothermic machine perfusion (NMP) to mimic in vitro conditions for effective gene delivery. In this study, lentiviral vectors encoding the secreted biomarker Gaussia Luciferase (GLuc) and red fluorescent protein (RFP) were introduced ex vivo to rodent livers during a 72-h machine perfusion protocol. After an initial 24-h exposure to viral vectors, the organs were maintained in perfusion for an additional 48 h to monitor gene expression, aligning with in vitro benchmarks. Control livers were perfused in similar fashion, but without viral injections. Virally perfused livers exhibited nearly a 10-fold increase in luminescence compared to controls (p < 0.0001), indicating successful genetic modification of the organs. These findings validate the use of machine perfusion systems and viral vectors to genetically engineer whole organs ex vivo, laying the groundwork for a broad range of applications in transplantation through genetic manipulation of organ systems. Future studies will focus on refining this technology to enhance precision in gene expression and explore its implications for clinical translation.
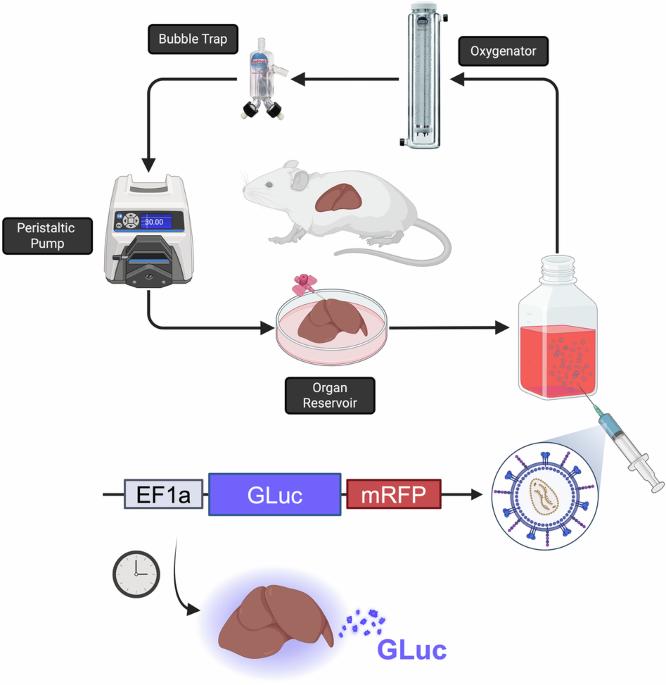
体外机器灌注作为慢病毒基因在大鼠肝脏传递的平台。
制定新的局部监测和提供免疫抑制的策略对于使同种异体移植更安全、更容易获得至关重要。利用机器灌注对移植物进行体外遗传修饰是一种很有前途的方法,可以改善移植物功能和调节免疫反应,同时最大限度地降低体内脱靶效应和全身免疫原性的风险。这项概念验证研究证明了使用常温机器灌注(NMP)模拟有效基因传递的体外条件的可行性。在这项研究中,慢病毒载体编码分泌的生物标志物高斯荧光素酶(GLuc)和红色荧光蛋白(RFP),在72小时的机器灌注过程中被体外引入啮齿动物肝脏。在初始暴露于病毒载体24小时后,器官在灌注中保持48小时以监测基因表达,与体外基准一致。对照肝脏以类似的方式灌注,但没有病毒注射。与对照组相比,病毒灌注的肝脏显示出近10倍的发光增加(p
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Gene Therapy
医学-生化与分子生物学
CiteScore
9.70
自引率
2.00%
发文量
67
审稿时长
4-8 weeks
期刊介绍:
Gene Therapy covers both the research and clinical applications of novel therapeutic techniques based on a genetic component. Over the last few decades, significant advances in technologies ranging from identifying novel genetic targets that cause disease through to clinical studies, which show therapeutic benefit, have elevated this multidisciplinary field to the forefront of modern medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


