Development of an AAV-RNAi strategy to silence the dominant variant GNAO1 c.607G>A linked to encephalopathy
IF 4.5
3区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
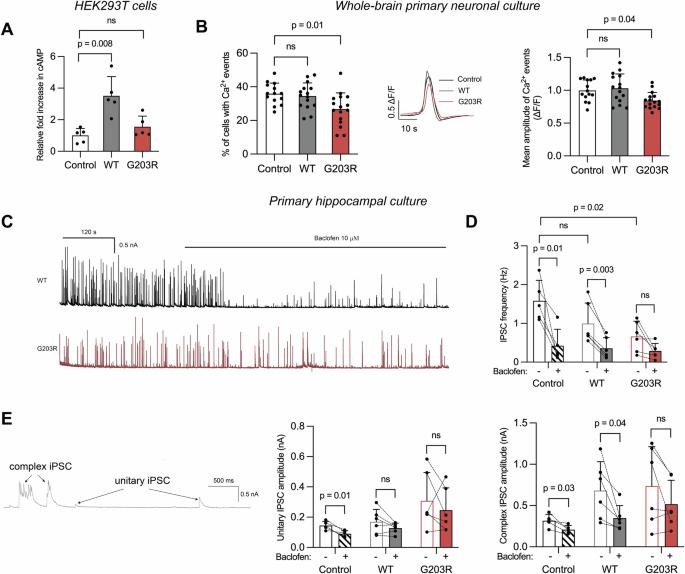
Heterozygous mutations in GNAO1 cause an ultra-rare neurodevelopmental disease called GNAO1 encephalopathy, characterized by infantile epilepsy and movement disorder. Here, we provide a functional characterization of the hotspot mutation GNAO1 c.607G>A (p.G203R) and conduct early-phase development of an adeno-associated virus (AAV)-mediated gene therapy approach. The GNAO1 gene encodes the Gαo protein that is involved in neuronal signaling. We showed that the Gαo-G203R lost its ability to enhance forskolin-stimulated cAMP synthesis in HEK293T cells. In primary neuronal culture, Gαo-G203R had a dominant-negative effect on neuronal activity and GABAB-dependent synaptic release. To ablate the mutant protein, we used selective silencing of the pathogenic variant using effectors of RNA interference (RNAi). We selected the short hairpin RNA (sh1500) that suppressed the c.607G>A transcripts, resulting in a 3.8-fold increase in the ratio of wild-type to mutant GNAO1 transcripts in patient-specific neurons. We also detected off-target effects of sh1500 as well as transcriptome changes associated with AAV transduction and RNAi activation. We improved the AAV construct by using an artificial miRNA (miR1500) and the neuron-specific hSyn promoter. Systemic administration of AAV9-hSyn-miR1500 did not cause pathological changes in Gnao1-GGA mice with a “humanized” target sequence. Importantly, AAV9 transduced Gαo-positive neurons in the striatum, thalamus, substantia nigra, and cerebellum, which we defined as primary targets for gene therapy. Our findings pave the road toward the development of AAV-RNAi approaches for dominant-negative GNAO1 variants.
开发一种AAV-RNAi策略来沉默与脑病相关的显性变异gnao1c . 607g >a。
GNAO1的杂合突变导致一种称为GNAO1脑病的超罕见神经发育疾病,以婴儿癫痫和运动障碍为特征。在这里,我们提供了热点突变GNAO1 c.607G> a (p.G203R)的功能特征,并进行了腺相关病毒(AAV)介导的基因治疗方法的早期开发。GNAO1基因编码参与神经元信号传导的Gαo蛋白。我们发现Gαo-G203R在HEK293T细胞中失去了增强福斯克林刺激的cAMP合成的能力。在原代神经元培养中,Gαo-G203R对神经元活性和gabab依赖性突触释放具有显性负向影响。为了清除突变蛋白,我们使用RNA干扰效应物(RNAi)选择性沉默致病变异。我们选择了短发夹RNA (sh1500)抑制c.607G>A转录本,导致患者特异性神经元中野生型与突变型GNAO1转录本的比例增加3.8倍。我们还检测到sh1500的脱靶效应以及与AAV转导和RNAi激活相关的转录组变化。我们使用人工miRNA (miR1500)和神经元特异性hSyn启动子来改进AAV结构。在具有“人源化”靶序列的Gnao1-GGA小鼠中,系统给药AAV9-hSyn-miR1500不会引起病理改变。重要的是,AAV9转导了纹状体、丘脑、黑质和小脑中的g αo阳性神经元,我们将其定义为基因治疗的主要靶点。我们的发现为开发针对GNAO1显性阴性变异的AAV-RNAi方法铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Gene Therapy
医学-生化与分子生物学
CiteScore
9.70
自引率
2.00%
发文量
67
审稿时长
4-8 weeks
期刊介绍:
Gene Therapy covers both the research and clinical applications of novel therapeutic techniques based on a genetic component. Over the last few decades, significant advances in technologies ranging from identifying novel genetic targets that cause disease through to clinical studies, which show therapeutic benefit, have elevated this multidisciplinary field to the forefront of modern medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


