The NET-DNA-CCDC25 inhibitor di-Pal-MTO suppresses tumor progression and promotes the innate immune response
IF 19.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
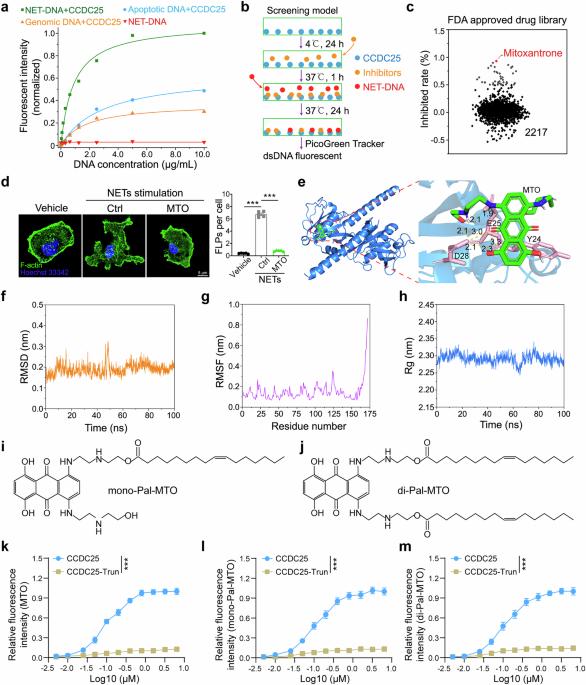
The DNA component of neutrophil extracellular traps (NET-DNA) is associated with cancer metastasis and chemotherapy resistance. However, recent studies have suggested that NET-DNA contributes to the activation of dendritic cells (DCs) and promotes the innate immune response to anticancer immunity. Therefore, exploring therapeutic approaches to inhibit NET-mediated tumor progression while maintaining antitumor immunity is essential. Our groups recently identified CCDC25 as a specific NET-DNA sensor on the cytoplasmic membrane of cancer cells that promotes cancer metastasis. In this study, we performed small-molecule compound screening and revealed that mitoxantrone (MTO) could block the interaction between NET-DNA and CCDC25. Molecular docking results indicated that MTO competed with NET-DNA by binding with the amino acid residues Tyr24 (Y24), Glu25 (E25), and Asp28 (D28) of the crystal structure of CCDC25. More importantly, we conjugated MTO with palmitoleic acids such as di-Pal-MTO to increase its residence time on the cytoplasmic membrane, which increased its inhibitory efficiency and decreased its cytotoxicity. In addition, di-Pal-MTO markedly inhibited the RAC1-CDC42 cascade to alleviate the NET-induced cytoskeleton arrangement and chemotactic migration of cancer cells. In multiple mouse models, di-Pal-MTO can suppress breast cancer metastasis and have synergistic effects with chemotherapeutics. Moreover, di-Pal-MTO promotes NET-DNA-dependent DC activation, leading to the subsequent expression of various chemokines that facilitate the infiltration of CD8+ T cells. Overall, we successfully identified a small molecule inhibitor, di-Pal-MTO, with dual effects on tumor repression and the antitumor immune response, which provides a novel therapeutic strategy against breast cancer.
NET-DNA-CCDC25抑制剂di-Pal-MTO抑制肿瘤进展并促进先天免疫反应。
中性粒细胞胞外陷阱(NET-DNA)的DNA成分与肿瘤转移和化疗耐药有关。然而,最近的研究表明,NET-DNA有助于激活树突状细胞(dc),并促进抗癌免疫的先天免疫反应。因此,在维持抗肿瘤免疫的同时,探索抑制net介导的肿瘤进展的治疗方法是至关重要的。我们的研究小组最近发现CCDC25是癌细胞细胞质膜上特异性的NET-DNA传感器,可促进癌症转移。在本研究中,我们进行了小分子化合物筛选,发现mitoxantrone (MTO)可以阻断NET-DNA与CCDC25的相互作用。分子对接结果表明,MTO通过结合CCDC25晶体结构的Tyr24 (Y24)、Glu25 (E25)和Asp28 (D28)氨基酸残基与NET-DNA竞争。更重要的是,我们将MTO与棕榈油酸(如di-Pal-MTO)偶联,增加了其在细胞质膜上的停留时间,从而提高了其抑制效率,降低了其细胞毒性。此外,di-Pal-MTO还能显著抑制RAC1-CDC42级联,减轻net诱导的癌细胞骨架排列和趋化迁移。在多种小鼠模型中,di-Pal-MTO可抑制乳腺癌转移,并与化疗药物具有协同作用。此外,di-Pal-MTO促进net - dna依赖性DC活化,导致随后各种趋化因子的表达,促进CD8+ T细胞的浸润。总之,我们成功鉴定了一种具有肿瘤抑制和抗肿瘤免疫反应双重作用的小分子抑制剂di-Pal-MTO,为乳腺癌的治疗提供了一种新的治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


