Activation and maturation of antigen-specific B cells in nonectopic lung infiltrates are independent of germinal center reactions in the draining lymph node
IF 19.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
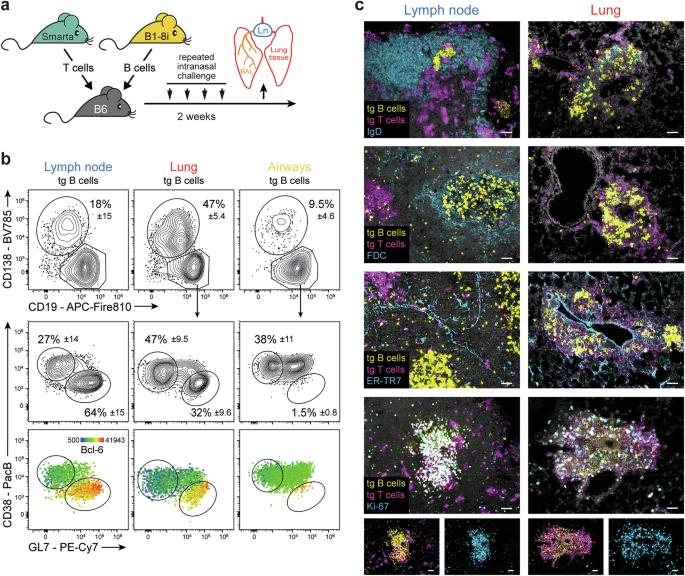
Pulmonary T and B cells are important for protection of this mucosal barrier site. While viral infections lead to the development of ectopic lymphoid structures highly similar to those in germinal centers in secondary lymphoid organs, little is known about how T/B cooperation occurs in the unstructured, diffuse tissue infiltrates characteristic of autoimmune diseases and nonviral infections. Using a mouse model of interstitial lung inflammation, we found that naive B cells are directly activated in lung tissue. Despite the absence of any germinal center-like structures, the interaction of B cells with peripheral T helper cells results in efficient somatic hypermutation and class switching. As antigen-presenting cells, macrophages are critical for this process. Unique B-cell repertoires indicated that the lung response was autonomous from the lung-draining lymph node. Only lung GC-like B cells were switched to IgA and had a broader repertoire, making them ideal candidates for producing broadly neutralizing immunoglobulins against respiratory pathogens.
非异位肺浸润中抗原特异性B细胞的激活和成熟与引流淋巴结的生发中心反应无关。
肺T细胞和B细胞对粘膜屏障部位的保护很重要。虽然病毒感染导致异位淋巴结构的发展与次级淋巴器官的生发中心高度相似,但对于自身免疫性疾病和非病毒感染特征的非结构化弥漫性组织浸润中T/B合作是如何发生的,我们知之甚少。利用小鼠肺间质性炎症模型,我们发现幼稚B细胞在肺组织中被直接激活。尽管没有任何生发中心样结构,但B细胞与外周辅助T细胞的相互作用导致有效的体细胞超突变和类别转换。巨噬细胞作为抗原呈递细胞,在这一过程中起着至关重要的作用。独特的b细胞谱表明肺反应是独立于肺引流淋巴结的。只有肺gc样B细胞被转换为IgA,并且具有更广泛的库,使它们成为产生广泛中和免疫球蛋白对抗呼吸道病原体的理想候选者。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


