HDAC1 is involved in the destabilization of the HSF2 protein under nonstress and stress conditions
IF 3.2
3区 生物学
Q3 CELL BIOLOGY
引用次数: 0
Abstract
Heat shock transcription factors 1 and 2 (HSF1 and HSF2) are the major regulators of the cellular response to stressors, notably to heat shock and to oxidative stress. HSF1 and HSF2 are also important contributors in devastating human pathologies like cancer, neurodegenerative disorders, and neurodevelopmental disorders. Under physiological conditions, nuclear HSF2 is detected in only a few cell types in human adult healthy tissues. In contrast, HSF2 protein levels are elevated at some embryonic stages, but greatly vary among cell types and fluctuate during the cell cycle in diverse cell lines. HSF2 is a short-lived protein whose rapid turnover is controlled by the components of the ubiquitin-proteasome degradation pathway, and the stabilization of HSF2 constitutes an important step that regulates its DNA-binding activity and mediates its roles in nonstress, physiological processes. The control of HSF2 abundancy is therefore critical for its regulatory roles in stress responses as well as under physiological conditions. In this regard, the fetal brain cortex is a singular context where HSF2 is strikingly abundant, exhibits constitutive DNA-binding activity and, by controlling a specific repertoire of target genes that play important roles at multiple steps of neurodevelopment. Recently, we showed that the lysine-acetyl-transferases CBP and EP300 stabilize the HSF2 protein under both unstressed and stressed conditions and that the integrity of the CBP/EP300-HSF2 pathway is important for neurodevelopment. Here, we identify the lysine-deacetylase histone-deacetylase 1 (HDAC1) as a novel HSF2-interacting protein partner and regulator, in an unbiased manner, and show that HSF2 and HDAC1 localize in the same cells in the developing mouse cortex and human cerebral organoids. We also demonstrate that HDAC1, through its catalytic activity, destabilizes the HSF2 protein, through HSF2 poly-ubiquitination and proteasomal degradation, under both normal and stress conditions.
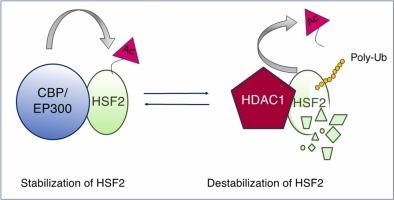
在非应激和应激条件下,HDAC1参与HSF2蛋白的不稳定。
热休克转录因子1和2 (HSF1和HSF2)是细胞对应激源,特别是热休克和氧化应激反应的主要调节因子。HSF1和HSF2也是癌症、神经退行性疾病和神经发育障碍等毁灭性人类疾病的重要贡献者。在生理条件下,人类成人健康组织中仅在少数细胞类型中检测到核HSF2。相反,HSF2蛋白水平在某些胚胎阶段升高,但在不同的细胞类型中差异很大,在不同细胞系的细胞周期中波动。HSF2是一种短寿命蛋白,其快速更新受泛素-蛋白酶体降解途径组分的控制,HSF2的稳定是调节其dna结合活性和介导其在非应激生理过程中的作用的重要步骤。因此,HSF2丰度的控制对于其在应激反应和生理条件下的调节作用至关重要。在这方面,胎儿大脑皮层是一个独特的环境,其中HSF2显著丰富,表现出组成性dna结合活性,并通过控制在神经发育的多个步骤中发挥重要作用的特定靶基因库。最近,我们发现赖氨酸-乙酰基转移酶CBP和EP300在非应激和应激条件下都能稳定HSF2蛋白,并且CBP/EP300-HSF2通路的完整性对神经发育很重要。在这里,我们以无偏倚的方式确定了赖氨酸去乙酰化酶HDAC1是一种新的HSF2相互作用蛋白伴侣和调节剂,并表明HSF2和HDAC1定位于发育中的小鼠皮层和人脑类器官(hCOs)的相同细胞中。我们还证明,在正常和应激条件下,HDAC1通过其催化活性,通过HSF2多泛素化和蛋白酶体降解,使HSF2蛋白不稳定。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Stress & Chaperones
生物-细胞生物学
CiteScore
7.60
自引率
2.60%
发文量
59
审稿时长
6-12 weeks
期刊介绍:
Cell Stress and Chaperones is an integrative journal that bridges the gap between laboratory model systems and natural populations. The journal captures the eclectic spirit of the cellular stress response field in a single, concentrated source of current information. Major emphasis is placed on the effects of climate change on individual species in the natural environment and their capacity to adapt. This emphasis expands our focus on stress biology and medicine by linking climate change effects to research on cellular stress responses of animals, micro-organisms and plants.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


