Regulation of nucleotide excision repair by wild-type HRAS signaling in head and neck cancer
IF 5
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
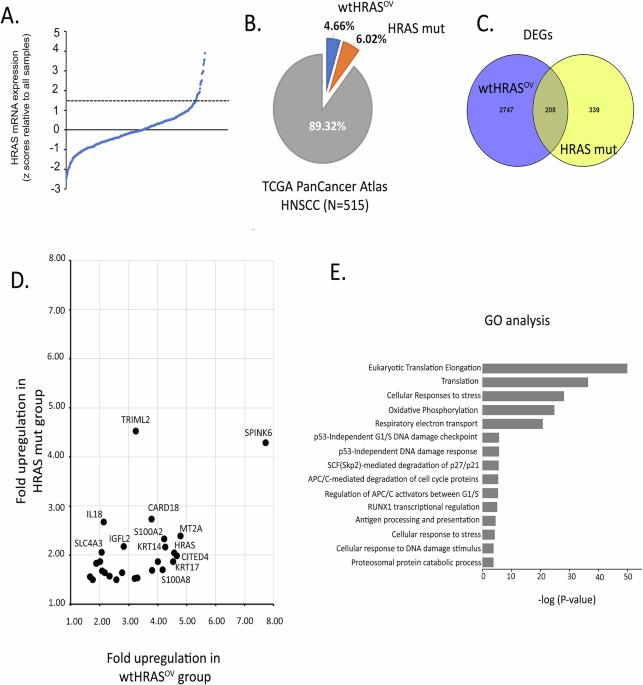
Head and neck squamous cell carcinoma (HNSCC) is characterized by a high rate of locoregional or distant relapse among patients. It is well established that resistance to chemotherapeutic drugs has an important role in the emergence of the recurrent and/or metastatic type of this malignancy which is associated with poor prognosis. Therefore, understanding the molecular basis of chemoresistance in head and neck cancer is required for the development of effective therapeutic strategies. Activating mutations in the HRAS gene are driver events in human cancer. Although numerous studies have demonstrated that oncogenic HRAS mutations promote chemoresistance in HNSCC, the molecular profile of HNSCC tumors that overexpress wild-type HRAS (wtHRASov) and their response to chemotherapy is poorly investigated. To gain deeper insights into the characteristics of wtHRASov tumors, we conducted a gene expression analysis using transcriptome data from The Cancer Genome Atlas (TCGA). This analysis revealed a distinct signature of overexpressed nucleotide excision repair (NER) genes in wtHRASov tumors, which are associated with chemoresistance. We further explored the role of these NER components in response to genotoxic stress, utilizing a diverse panel of HNSCC cell lines and patient-derived xenografts. Our findings indicate that in a specific cluster of head and neck tumors, ERK/cJun signaling activation is strongly reliant on HRAS activity. Inhibiting HRAS in these tumors results in a significant downregulation of the NER signature components, re-sensitizing cancer cells to platinum-based chemotherapy.
野生型HRAS信号在头颈癌中对核苷酸切除修复的调控。
头颈部鳞状细胞癌(HNSCC)的特点是患者的局部或远处复发率高。众所周知,对化疗药物的耐药性在这种恶性肿瘤的复发和/或转移类型的出现中起着重要作用,这种恶性肿瘤与预后不良有关。因此,了解头颈癌化疗耐药的分子基础是制定有效治疗策略的必要条件。HRAS基因的激活突变是人类癌症的驱动事件。尽管大量研究表明,致瘤性HRAS突变促进HNSCC的化疗耐药,但对过表达野生型HRAS (wtHRASov)的HNSCC肿瘤的分子谱及其对化疗的反应研究甚少。为了更深入地了解wtHRASov肿瘤的特征,我们使用来自癌症基因组图谱(TCGA)的转录组数据进行了基因表达分析。该分析揭示了wtHRASov肿瘤中过表达的核苷酸切除修复(NER)基因的明显特征,这与化疗耐药有关。我们利用不同的HNSCC细胞系和患者来源的异种移植物进一步探索了这些NER成分在基因毒性应激反应中的作用。我们的研究结果表明,在特定的头颈部肿瘤簇中,ERK/cJun信号激活强烈依赖于HRAS活性。在这些肿瘤中抑制HRAS会导致NER特征成分的显著下调,使癌细胞对铂类化疗重新敏感。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


