Lactate-coated polyurea-siRNA dendriplex: a gene therapy-directed and metabolism-based strategy to impair glioblastoma (GBM)
IF 5
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
Glioblastoma (GBM) is a highly lethal disease with limited treatment options due to its infiltrative nature and the lack of efficient therapy able to cross the protective blood-brain barrier (BBB). GBMs are metabolically characterized by increased glycolysis and glutamine dependence. This study explores a novel metabolism-based therapeutic approach using a polyurea generation 4 dendrimer (PUREG4) surface functionalized with lactate (LA) (PUREG4-LA24), to take advantage of glucose-dependent monocarboxylate transporters (MCTs) overexpression, loaded with selenium-chrysin (SeChry) and temozolomide (TMZ) or complexed with anti-glutaminase (GLS1) siRNAs to abrogate glutamine dependence. The nanoparticles (PUREG4-LA24) were efficient vehicles for cytotoxic compounds delivery, since SeChry@PUREG4-LA24 and TMZ@PUREG4-LA24 induced significant cell death in GBM cell lines, particularly in U251, which exhibits higher MCT1 expression. The anti-GLS1 siRNA-dendriplex with PUREG4-LA12 (PUREG4-LA12-anti-GLS1-siRNA) knocked down GLS1 in the GBM cell lines. In two in vitro BBB models, these dendriplexes successfully crossed the BBB, decreased GLS1 expression and altered the exometabolome of GBM cell lines, concomitantly with autophagy activation. Our findings highlight the potential of targeting glucose and glutamine pathways in GBM using dendrimer-based nanocarriers, overcoming the BBB and disrupting key metabolic processes in GBM cells.
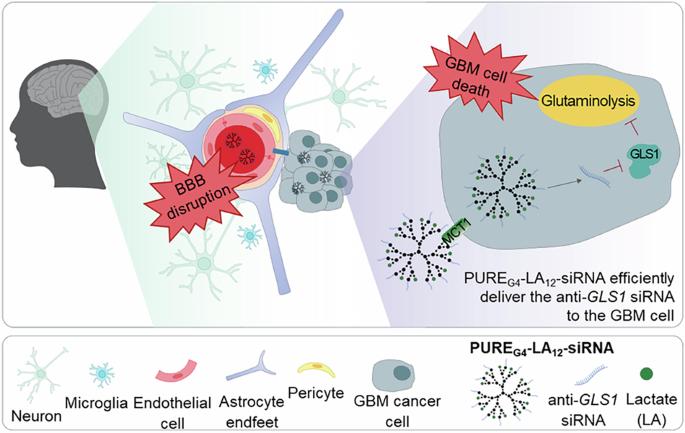
乳酸包被聚氨酯- sirna树突:一种基因治疗导向和基于代谢的策略,以损害胶质母细胞瘤(GBM)。
胶质母细胞瘤(GBM)是一种高度致命的疾病,由于其浸润性和缺乏能够穿过保护性血脑屏障(BBB)的有效治疗方法,治疗方案有限。GBMs的代谢特征是糖酵解和谷氨酰胺依赖性增加。本研究探索了一种新的基于代谢的治疗方法,使用聚脲第4代树状大分子(PUREG4)表面被乳酸(LA)功能化(PUREG4- la24),利用葡萄糖依赖的单羧酸转运体(mct)过表达,负载硒-chrysin (SeChry)和替莫唑胺(TMZ)或与抗谷氨酰胺酶(GLS1) sirna络合来消除谷氨酰胺依赖性。纳米颗粒(PUREG4-LA24)是细胞毒性化合物递送的有效载体,因为SeChry@PUREG4-LA24和TMZ@PUREG4-LA24在GBM细胞系中诱导了显著的细胞死亡,特别是在MCT1表达较高的U251中。具有PUREG4-LA12的抗GLS1 sirna树突(PUREG4-LA12-anti-GLS1- sirna)在GBM细胞系中敲低GLS1。在两种体外血脑屏障模型中,这些树突成功穿过血脑屏障,降低GLS1表达,改变GBM细胞系的外代谢组,并伴有自噬激活。我们的研究结果强调了利用基于树突的纳米载体靶向GBM中葡萄糖和谷氨酰胺通路的潜力,克服了血脑屏障并破坏了GBM细胞中的关键代谢过程。PUREG4-LA12-anti-GLS1-siRNA树突穿过血脑屏障(BBB)并损害胶质母细胞瘤(GBM)代谢。血脑屏障是由一层薄薄的特化的大脑微血管内皮细胞通过紧密连接连接在一起形成的,这些细胞有选择地控制物质从血液到大脑的通道。这是治疗GBM的主要障碍,因为许多化疗药物无法穿透大脑。因此,我们开发了一种克服这一障碍的策略:一种乳酸包被的聚脲树状大分子第4代(PUREG4),能够在体外穿过血脑屏障,作为药物和siRNA的纳米载体进入GBM细胞。PUREG4-LA12是用乳酸(LA)功能化的纳米颗粒,靶向MCT1,一种由GBM细胞高度表达的乳酸转运蛋白。此外,制备了该纳米颗粒与抗gls1(谷氨酰胺酶)siRNA的复合物(PUREG4-LA12-anti-GLS1-siRNA),以靶向谷氨酰胺代谢。它有效地击倒了GLS1。此外,PUREG4-LA24加载secry导致BBB中断。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


