Uncovering the Unique Roles of Cathepsins B and L in Purkinje Cells Using Nervous System–Specific CTSB and CTSL Double-Deficient Mice
IF 3.6
2区 医学
Q1 PATHOLOGY
引用次数: 0
Abstract
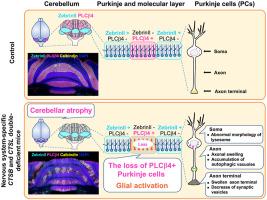
Defects in the autophagy-lysosomal degradation pathway contribute to neurodegenerative diseases. Cathepsins B (CtsB) and L (CtsL) are major lysosomal cysteine proteases. Mice deficient in both CtsB and CtsL exhibit abnormalities not only in neural tissue but in other organs, such as heart and liver, with most dying at approximately 16 days of age. To investigate the roles of CtsB and CtsL in the nervous system, nervous system–specific CTSB and CTSL double-knockout mice (CtsB/L-NES: CTSBflox/flox; CTSLflox/flox; Nestin-Cre) were generated. These mice displayed tail elevation, motor dysfunction, and hyperactivity. In adulthood, they developed cerebellar atrophy, with Purkinje cells in the cerebellar cortex showing selective loss of phospholipase C β4–positive and Zebrin II–negative Purkinje cells in a striped pattern. Ubiquitin-positive structures accumulated in the perikarya and axons of Purkinje cells, reflecting impaired autophagy-lysosomal degradation. Activation of astrocytes and microglia was observed in Purkinje cell loss regions. Electron microscopy revealed granular osmiophilic deposit-like structures in perikarya, autophagosome-like accumulations in axons, and a reduction in synaptic vesicles at active regions of swollen axon terminals in Purkinje cells. Additionally, neuronal loss was observed specifically in the ventral posterior lateral and ventral posterior medial nuclei of the thalamus. These findings demonstrate CtsB and CtsL are essential for survival of Purkinje cells in the cerebellum and neurons in the ventral posterior lateral and ventral posterior medial nuclei of the thalamus.
利用神经系统特异性CTSB和CTSL双缺陷小鼠揭示组织蛋白酶B和L在浦肯野细胞中的独特作用
自噬-溶酶体降解途径的缺陷导致神经退行性疾病。组织蛋白酶B (CtsB)和L (CtsL)是主要的溶酶体半胱氨酸蛋白酶。缺乏CtsB和CtsL的小鼠不仅在神经组织中表现出异常,而且在心脏和肝脏等其他器官中也表现出异常,大多数小鼠在16天左右死亡。为了研究CtsB和CtsL在神经系统中的作用,我们制造了神经系统特异性CtsB和CtsL双敲除小鼠(CtsB/L-NES)。这些小鼠表现出尾巴抬高、运动功能障碍和多动症。成年后,他们出现了小脑萎缩,小脑皮层的浦肯野细胞显示出选择性的磷脂酶C β4阳性和Zebrin ii阴性浦肯野细胞的条纹状损失。泛素阳性结构积聚在浦肯野细胞核周和轴突,反映自噬-溶酶体降解受损。星形胶质细胞和小胶质细胞在浦肯野细胞丢失区被激活。电镜显示浦肯野细胞核周有颗粒状亲锇沉积物样结构,轴突有自噬体样堆积,肿胀的轴突末端活跃区域突触囊泡减少。此外,在丘脑腹侧后外侧核(VPL)和腹侧后内侧核(VPM)中观察到神经元损失。这些发现表明CtsB和CtsL对于小脑浦肯野细胞和丘脑VPL和VPM神经元的存活至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.40
自引率
0.00%
发文量
178
审稿时长
30 days
期刊介绍:
The American Journal of Pathology, official journal of the American Society for Investigative Pathology, published by Elsevier, Inc., seeks high-quality original research reports, reviews, and commentaries related to the molecular and cellular basis of disease. The editors will consider basic, translational, and clinical investigations that directly address mechanisms of pathogenesis or provide a foundation for future mechanistic inquiries. Examples of such foundational investigations include data mining, identification of biomarkers, molecular pathology, and discovery research. Foundational studies that incorporate deep learning and artificial intelligence are also welcome. High priority is given to studies of human disease and relevant experimental models using molecular, cellular, and organismal approaches.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


