Harnessing nutrient scarcity for enhanced CAR-T-cell potency and safety in solid tumors
IF 19.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
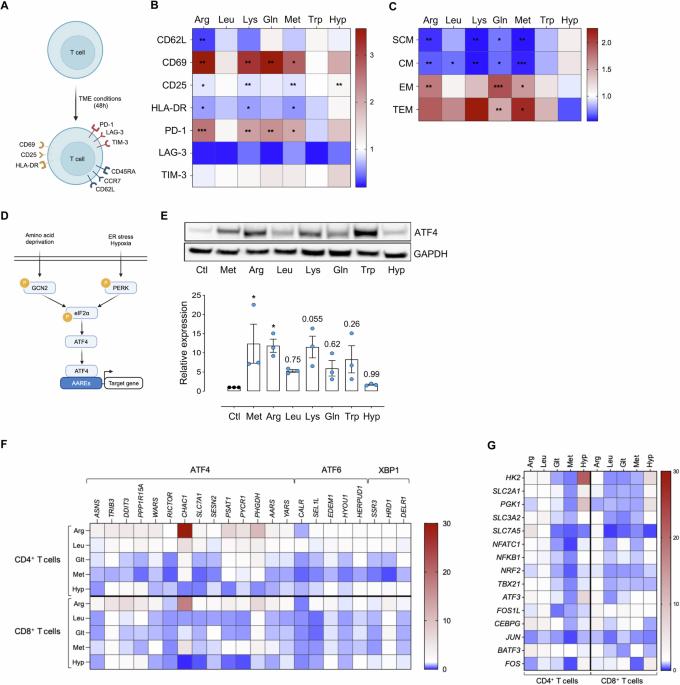
Despite significant advancements, the effectiveness of chimeric antigen receptor (CAR)-T-cell-based therapies in solid tumors remains limited. Key challenges include on-target effects, off-tumor toxicity and reduced CAR-T-cell function within the tumor microenvironment, which is often characterized by metabolic stress triggered by factors such as amino acid scarcity. Activating transcription factor-4 (ATF4) and its upstream regulator GCN2 play crucial roles in the metabolic reprogramming and functionality of CD4+ and CD8+ T cells. ATF4 can be activated by various cellular stress signals, including amino acid deprivation. While ATF4 activation may be associated with T-cell dysfunction, its role in stress adaptation presents an opportunity for therapeutic intervention—particularly in the tumor microenvironment, where T-cell exhaustion is a major challenge. In this study, we developed a strategy to harness the GCN2‒ATF4 axis in CAR-T cells. We employed an amino acid-dependent inducible promoter, which triggers ATF4-dependent gene expression to regulate CAR expression in T cells under conditions of amino acid scarcity within the tumor microenvironment. In vitro and murine xenograft models demonstrate the potential of this system to effectively restrict CAR expression to the tumor site. This targeted strategy not only enhances safety by minimizing off-tumor activity but also CAR-T-cell fitness by reducing exhaustion. By validating this pathophysiologically regulatable CAR expression system for solid tumors, our findings address key limitations of current CAR-T-cell therapies and pave the way for innovative strategies targeting solid malignancies.
利用营养稀缺增强car - t细胞在实体肿瘤中的效力和安全性。
尽管取得了重大进展,嵌合抗原受体(CAR)- t细胞治疗实体瘤的有效性仍然有限。关键挑战包括靶效应、肿瘤外毒性和肿瘤微环境中car -t细胞功能降低,其特征通常是由氨基酸缺乏等因素引发的代谢应激。激活转录因子-4 (ATF4)及其上游调控因子GCN2在CD4+和CD8+ T细胞的代谢重编程和功能中起着至关重要的作用。ATF4可被多种细胞应激信号激活,包括氨基酸剥夺。虽然ATF4激活可能与t细胞功能障碍有关,但它在应激适应中的作用为治疗干预提供了机会,特别是在肿瘤微环境中,t细胞衰竭是一个主要挑战。在这项研究中,我们开发了一种在CAR-T细胞中利用GCN2-ATF4轴的策略。我们使用氨基酸依赖的诱导启动子,在肿瘤微环境中氨基酸缺乏的条件下,触发atf4依赖的基因表达来调节T细胞中CAR的表达。体外和小鼠异种移植模型证明了该系统有效限制CAR在肿瘤部位表达的潜力。这种有针对性的策略不仅通过最小化肿瘤外活性来提高安全性,而且通过减少衰竭来提高car -t细胞的适应性。通过验证这种病理生理可调节的CAR在实体肿瘤中的表达系统,我们的发现解决了当前CAR- t细胞疗法的关键局限性,并为针对实体恶性肿瘤的创新策略铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


