Glioblastoma-associated macrophages in glioblastoma: from their function and mechanism to therapeutic advances
IF 5
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
Glioblastoma multiforme (GBM) is the most aggressive primary brain tumor in adults and has high mortality rates worldwide. GBM progression, treatment, and prognosis are influenced by the tumor microenvironment (TME), which includes immune, stromal, and tumor cells. Among them, glioblastoma-associated macrophages (GAMs) act as key regulators of GBM pathobiology. GAMs exhibit remarkable plasticity, as they can exhibit both protumor and antitumor effects. However, their function is determined by polarization and the TME. In this review, we provide a comprehensive overview of the current understanding of the biology of GAMs in GBM, including their origins, phenotypic diversity, and functional roles. We discuss the intricate crosstalk between GAMs and tumor cells, as well as other immune and stromal components, and highlight the mechanisms underlying GAM-mediated tumor progression, invasion, angiogenesis, and immune system evasion. Furthermore, we explore the therapeutic implications of targeting GAMs in GBM and discuss emerging strategies aimed at reprogramming GAMs toward an antitumorigenic phenotype or selectively depleting protumorigenic subsets. The final aim is to develop innovative therapeutic approaches that disrupt GBMs. By leveraging our increased understanding of GAM biology, we lay the foundation for transformative advances in GBM treatment to improve patient prognosis.
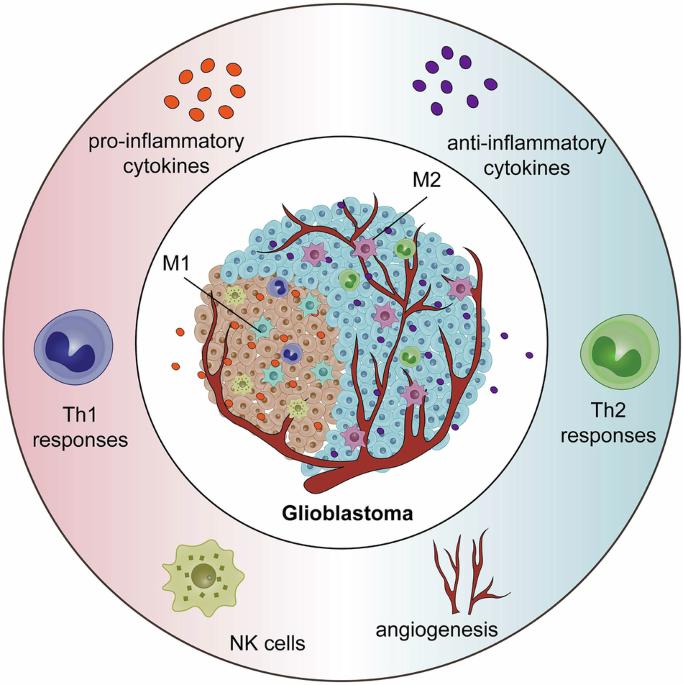
胶质母细胞瘤相关巨噬细胞:从功能、机制到治疗进展。
多形性胶质母细胞瘤(GBM)是成人中最具侵袭性的原发性脑肿瘤,在世界范围内具有很高的死亡率。GBM的进展、治疗和预后受肿瘤微环境(tumor microenvironment, TME)的影响,包括免疫细胞、基质细胞和肿瘤细胞。其中,胶质母细胞瘤相关巨噬细胞(GAMs)是GBM病理生物学的关键调节因子。GAMs具有显著的可塑性,可以同时表现出抗肿瘤和抗肿瘤的作用。然而,它们的功能是由极化和TME决定的。在这篇综述中,我们全面概述了目前对GBM中GAMs生物学的理解,包括它们的起源、表型多样性和功能作用。我们讨论了GAMs和肿瘤细胞之间复杂的串扰,以及其他免疫和基质成分,并强调了GAMs介导的肿瘤进展、侵袭、血管生成和免疫系统逃避的机制。此外,我们探讨了靶向GAMs在GBM中的治疗意义,并讨论了旨在将GAMs重编程为抗肿瘤表型或选择性地消耗致瘤亚群的新兴策略。最终目标是开发创新的治疗方法来破坏GBMs。通过利用我们对GAM生物学的深入了解,我们为GBM治疗的变革性进步奠定了基础,以改善患者预后。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


