Synthesis of novel derivatives from machilin C/D as antiproliferative agents against triple negative breast cancer
European Journal of Medicinal Chemistry Reports
Pub Date : 2025-05-08
DOI:10.1016/j.ejmcr.2025.100272
引用次数: 0
Abstract
Lignans are small polyphenolic compounds that play an active role in plant defense against pathogens and predators. Recently, lignan machilin D was reported to be effective as an anti-tumorigenic agent in triple negative breast cancer (TNBC) tumor-bearing mice. Previous studies have relied on plant-extracted material limiting scalability and diversification of the natural scaffold. Herein, we describe a generalizable one-pot synthesis of machilin D and its derivatives via an iron chloride-induced dimerization of isoeugenol. Employing this synthetic methodology allowed for a robust diversification campaign to access seven (7) new lignan derivatives of the machilin D family with superior anti-proliferative properties in 2D and 3D TNBC models. Overall, this work enables lignan natural product-based drug discovery as a platform to identify new probes to elucidate lignan targets in biology and therapeutics for aggressive cancers such as TNBC.
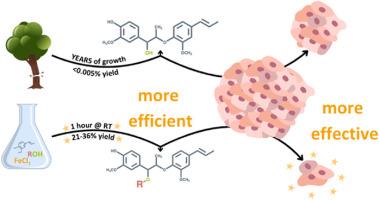
抗三阴性乳腺癌的新衍生物machilin C/D的合成
木脂素是一种小的多酚类化合物,在植物防御病原体和捕食者中起着积极的作用。最近,木脂素machilin D作为一种抗肿瘤药物被报道在三阴性乳腺癌(TNBC)荷瘤小鼠中有效。以前的研究依赖于植物提取材料,限制了天然支架的可扩展性和多样性。在这里,我们描述了一锅通过氯化铁诱导异丁香酚二聚化合成machilin D及其衍生物的一般方法。采用这种合成方法,可以在2D和3D TNBC模型中获得七(7)种新的木脂素衍生物machilin D家族,具有优越的抗增殖特性。总的来说,这项工作使木脂素天然产物为基础的药物发现提供了一个平台,以确定新的探针来阐明木脂素在生物学和治疗侵袭性癌症(如TNBC)中的靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


