Anti-tumor and cellular mechanisms of HfIV tetra-(8-hydroxyquinolinato) complexes
IF 3.2
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Three 8-hydroxyquinoline-stabilized hafnium complexes, [HfIV(oxinate)4], were synthesized with good aqueous stability and solubility by reacting HfIVCl4 with 8-hydroxyquinoline (HL1), 2-methyl-8-hydroxyquinoline (HL2) and 5-chloro-8-hydroxyquinoline (HL3) in THF, achieving high yields. Among the synthesized complexes, [HfIV(L1)4] and [HfIV(L3)4] exhibited potent inhibitory activity against human liver (Hep G2), cervical (HeLa S3) and lung (PC9) cancer cell lines, while showing low toxicity against non-tumorigenic murine epithelial AML12 cells. Notably, [HfIV(L1)4] demonstrated the most potent activity, with an IC50 value of 0.8 ± 0.3 μM against Hep G2 cells, which is 17 times lower than that of cisplatin (IC50 = 13.8 ± 1.3 μM). Mechanistic cell studies revealed that [HfIV(L1)4] could effectively inhibit cell migration, induce reactive oxygen species generation and cause mitochondrial membrane potential disruption. Furthermore, [HfIV(L1)4] blocked the cell cycle progression at the G2/M phase and led almost exclusively to early apoptosis in Hep G2 cells. Western blot analysis revealed that in Hep G2 cells [HfIV(L1)4] could upregulate the expression of caspase-3 and Bax proteins while downregulating the expression of the anti-apoptotic Bcl-2 protein, highlighting the apoptotic pathway as a key mechanism of action. Comparisons are made with previously reported [ZrIV(L1)4], which shows higher cytotoxicity, cellular uptake, reactive oxygen species generation, mitochondrial damage and stronger inhibition of antioxidant enzymes' activity. However, [HfIV(L1)4] induces primarily early apoptosis, which is advantageous. Overall, these rare earth complexes, particularly [HfIV(L1)4] and [ZrIV(L1)4], demonstrate promising potential as novel anticancer agents with significant efficacy against human liver cancer cells and favourable selectivity profiles for further therapeutic development.
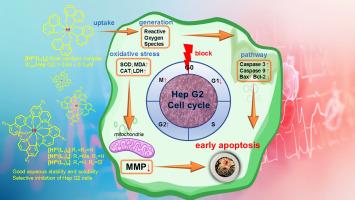
HfIV四-(8-羟基喹啉酸)复合物的抗肿瘤和细胞机制
通过HfIVCl4与8-羟基喹啉(HL1)、2-甲基-8-羟基喹啉(HL2)和5-氯-8-羟基喹啉(HL3)在THF中反应,合成了3个8-羟基喹啉稳定的铪配合物[HfIV(氧酸盐)4],具有良好的水稳定性和溶解度,收率较高。在合成的复合物中,[HfIV(L1)4]和[HfIV(L3)4]对人肝癌(Hep G2)、宫颈癌(HeLa S3)和肺癌(PC9)细胞系表现出强抑制活性,而对非致瘤性小鼠上皮AML12细胞表现出低毒性。值得注意的是,[HfIV(L1)4]表现出最强的活性,对Hep G2细胞的IC50值为0.8±0.3 μM,比顺铂低17倍(IC50 = 13.8±1.3 μM)。细胞机制研究表明,[HfIV(L1)4]能有效抑制细胞迁移,诱导活性氧的产生,并引起线粒体膜电位破坏。此外,[HfIV(L1)4]在G2/M期阻断细胞周期进程,几乎完全导致Hep G2细胞的早期凋亡。Western blot分析显示,在Hep G2细胞中[HfIV(L1)4]可上调caspase-3和Bax蛋白的表达,下调抗凋亡Bcl-2蛋白的表达,提示凋亡通路是其关键作用机制。与先前报道的[ZrIV(L1)4]进行比较,显示出更高的细胞毒性、细胞摄取、活性氧生成、线粒体损伤和更强的抗氧化酶活性抑制。然而,[HfIV(L1)4]主要诱导早期细胞凋亡,这是有利的。总的来说,这些稀土配合物,特别是[HfIV(L1)4]和[ZrIV(L1)4],作为一种新的抗癌药物,具有很大的潜力,对人类肝癌细胞具有显著的疗效,并具有良好的选择性,可用于进一步的治疗开发。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


