Rhodium-catalyzed one-pot tandem reductive amination/asymmetric transfer hydrogenation of quinoxaline-2-carbaldehydes and anilines for the efficient synthesis of chiral vicinal diamines†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-04-26
DOI:10.1039/d5qo00625b
引用次数: 0
Abstract
Enantioselective synthesis of chiral N,N′-diaryl vicinal diamines via Cp*Rh-catalyzed one-pot tandem reductive amination/asymmetric transfer hydrogenation of quinoxaline-2-carbaldehydes with anilines using HCO2H/NEt3 (5/2) as the reductant has been developed. This mild catalytic system exhibits broad substrate generality and efficiently produces diversely substituted chiral N,N′-diaryl vicinal diamines with opposite configurations in high yields and excellent enantioselectivities (up to >99% ee). The synthetic utility of this protocol has been validated through successful gram-scale synthesis and facile derivatization of the resulting chiral vicinal diamines.
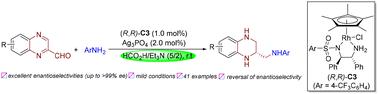

铑催化喹啉-2-乙醛和苯胺的一锅串联还原胺化/不对称转移加氢高效合成手性邻二胺
以HCO2H/NEt3(5/2)为还原剂,采用Cp* rh催化喹啉-2-乙醛与苯胺的一锅连续还原胺化/不对称转移加氢,对映选择性合成了手性N,N ' -二芳基邻二胺。这种温和的催化体系具有广泛的底物通用性,并能高效地产生不同取代的手性N,N ' -二芳基邻二胺,具有相反的构型,产率高,对映选择性好(高达99% ee)。该方案的合成效用已通过成功的克级合成和易于衍生的手性邻胺得到验证。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


