A General Medium-to-Large Sized Ring Synthesis Enabled by Copper-Catalyzed Difluoroalkylamidation Cyclization of Alkynes
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
This paper describes a novel coordinating activation strategy that enables the synthesis of medium-to-large sized rings (11–17 members) via an unprecedented difluoroalkylamidation cyclization of alkynes. This method provides an efficient platform for accessing skeleton-diverse difluoroalkyl-containing cyclic enamides with complete regio- and stereoselectivity. The protocol features broad substrate compatibility, functional group tolerance, and ease of use at dilution concentrations (50 mM) that are not high. Moreover, the synthetic utility of this difunctional cyclization is underscored by its application in the late-stage modification of complex molecules. Additionally, the click reaction facilitates the derivation of alkynyl-substituted cyclization products, demonstrating the methodology’s potential in biological sciences.
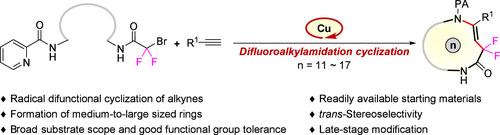
铜催化炔烃的二氟烷基酰胺化环化合成中大型环
本文描述了一种新的配位活化策略,通过前所未有的炔的二氟烷基酰胺环化,可以合成中大型环(11-17个成员)。该方法为获得具有完全区域选择性和立体选择性的骨架多样化含二氟烷基环酰胺提供了一个有效的平台。该方案具有广泛的底物相容性,官能团耐受性,以及在不高稀释浓度(50 mM)下易于使用的特点。此外,这种双官能团环化在复杂分子的后期修饰中的应用强调了它的合成效用。此外,点击反应促进了烷基取代环化产物的衍生,证明了该方法在生物科学中的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


