Access to ester-substituted γ-butyrolactones via photocatalyzed alkoxycarbonylation/lactonization of alkenoic acids†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-04-26
DOI:10.1039/d5qo00520e
引用次数: 0
Abstract
We reported a versatile and concise method to realize the photoredox-induced alkoxycarbonylative lactonization of alkenoic acids for the construction of ester-substituted γ-butyrolactones. This method achieves a multistep cascade reaction by combining photoinduced alkoxycarbonyl radical addition, single-electron oxidization, and lactonization. The reaction shows good compatibility of functional groups with a diverse range of alkoxycarbonyl radical precursors, enabling the efficient introduction of an ester group into the γ-butyrolactone scaffold.
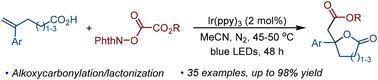
通过光催化烯酸烷氧羰基化/内酯化制备酯取代γ-丁内酯
我们报道了一种通用的、简洁的方法来实现光氧化还原诱导的烯酸烷氧羰基内酯化,以构建酯取代的γ-丁内酯。该方法通过光诱导烷氧羰基自由基加成、单电子氧化和内酯化反应实现了多步级联反应。该反应显示出官能团与多种烷氧羰基自由基前体具有良好的相容性,能够有效地将酯基引入到γ-丁内酯支架中。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


