Catalytic Atroposelective aza-Grob Fragmentation: An Approach toward Axially Chiral Biarylnitriles
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Grob fragmentation is a powerful synthetic tool for cleaving C–C bonds, which was particularly useful in the construction of seven- to nine-membered carbocycles or heterocycles. This reaction typically breaks one C–C bond and one C–X bond and forms two unsaturated functional groups. As no stereogenic centers are generated, catalytic asymmetric Grob fragmentation has remained unexplored. In this study, we have successfully developed a catalytic asymmetric aza-Grob fragmentation of α-keto oxime esters, achieving atroposelective C–C bond cleavage to construct axially chiral biarylnitriles. Single-crystal X-ray diffraction analysis of oxime esters elucidated the structure–reactivity relationship, highlighting the role of torsional strain. These studies also revealed the unique role of the 2-phenyl benzoyl group in controlling the substrate conformation, tuning reactivity, and stereoselectivity. The 1H NMR titration experiments provided brief insights into the activation mode of the catalyst with the substrate, suggesting a multi-hydrogen-bonding interaction model.
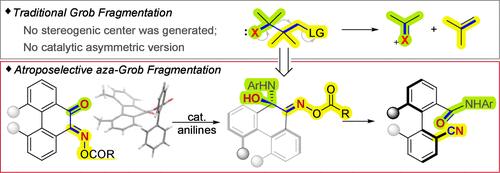
催化atroopselective aza-Grob裂解:轴向手性双芳基腈的研究
Grob断裂是一种切割C-C键的强大合成工具,在构建七至九元碳环或杂环时特别有用。这个反应通常会破坏一个C-C键和一个C-X键,形成两个不饱和官能团。由于没有生成立体中心,催化不对称Grob断裂仍未被探索。在这项研究中,我们成功地开发了α-酮肟酯的催化不对称aza-Grob断裂,实现了atroopselective C-C键裂解,构建了轴手性双芳基腈。单晶x射线衍射分析表明了肟酯的结构-反应性关系,强调了扭转应变的作用。这些研究还揭示了2-苯基苯甲酰在控制底物构象、调节反应活性和立体选择性方面的独特作用。1H NMR滴定实验提供了催化剂与底物的激活模式的简要见解,提出了一个多氢键相互作用模型。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


