Unraveling the Mechanism of Higher Shock Sensitivity Induced by Rapid Reactions of the Azoxy Group
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Azoxy groups in explosive molecules usually act as energy enhancers but also as potential sensitivity modulators. 3,3′-Diamino-4,4’-azoxyfurazan (DAAF) is a high-nitrogen explosive characterized by the presence of an azoxy group. It exhibits relatively insensitive characteristics to mechanical stimuli such as impact and friction while also possessing characteristics of small critical diameter and sensitivity to specific shock stimuli. To gain a deeper understanding of these properties, the reactive force field parameters for DAAF were optimized, and reactive molecular dynamics (RMD) simulations were employed to study the reaction process of DAAF under shock loading. Meanwhile, the electrostatic potential (ESP) on the van der Waals surface and the bond dissociation energy of several structures were calculated by density functional theory (DFT). Then, reaction mechanisms were analyzed, and their correlations with sensitivity were explored. Results revealed that DAAF exhibits cluster evolution characteristics similar to those of TATB. However, the unique azoxy group in DAAF plays a crucial role in the initial reactions under shock loading. Key initial reactions, such as polymerization and oxygen transfer, primarily occur around the oxygen atoms in the azoxy group. After shock-induced polymerization, the dissociation energies of the chemical bonds associated with the azoxy group in DAAF molecules significantly decrease. The shock-initiated rapid reactions of the azoxy group are the important reason for DAAF’s sensitivity to shock stimuli. This suggests that the sensitivity characteristics of explosives can be modulated by introducing appropriate functional groups into the molecular structure, making them sensitive to specific stimuli.
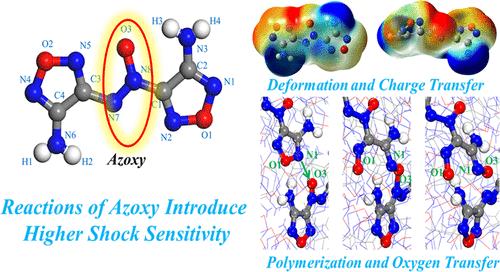
偶氮基快速反应诱导高休克敏感性的机制揭示
偶氮基在炸药分子中通常作为能量增强剂,但也作为潜在的灵敏度调节剂。3,3 ' -二氨基-4,4 ' -偶氮呋喃氮(DAAF)是一种高氮炸药,其特征是存在一个偶氮基。它对冲击和摩擦等机械刺激表现出相对不敏感的特性,同时也具有临界直径小和对特定冲击刺激敏感的特性。为了更深入地了解这些特性,优化了DAAF的反应力场参数,并采用反应分子动力学(RMD)模拟研究了DAAF在冲击载荷下的反应过程。同时,利用密度泛函理论(DFT)计算了几种结构的范德华表面静电势(ESP)和键解离能。然后,分析了反应机理,并探讨了其与敏感性的相关性。结果表明,DAAF具有与TATB相似的集群进化特征。然而,DAAF中独特的偶氮基在冲击载荷下的初始反应中起着至关重要的作用。关键的初始反应,如聚合和氧转移,主要发生在偶氮基的氧原子周围。经冲击聚合后,DAAF分子中偶氮基相关化学键的离解能显著降低。偶氮氧基组休克引发的快速反应是DAAF对休克刺激敏感的重要原因。这表明炸药的敏感特性可以通过在分子结构中引入适当的官能团来调节,使其对特定刺激敏感。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


